NDC 63029-664
GOODYS Extra Strength Headache Mixed Fruit Blast
Acetaminophen, Aspirin, And Caffeine
GOODYS Extra Strength Headache Mixed Fruit Blast is a Oral Powder in the Human Otc Drug category. It is labeled and distributed by Medtech Products Inc.. The primary component is Caffeine; Acetaminophen; Aspirin.
| Product ID | 63029-664_061df2d8-dea3-4024-8546-6bbdbf37c81a |
| NDC | 63029-664 |
| Product Type | Human Otc Drug |
| Proprietary Name | GOODYS Extra Strength Headache Mixed Fruit Blast |
| Generic Name | Acetaminophen, Aspirin, And Caffeine |
| Dosage Form | Powder |
| Route of Administration | ORAL |
| Marketing Start Date | 2015-08-01 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Labeler Name | Medtech Products Inc. |
| Substance Name | CAFFEINE; ACETAMINOPHEN; ASPIRIN |
| Active Ingredient Strength | 65 mg/1; mg/1; mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 63029-664-04
4 POWDER in 1 CARTON (63029-664-04)
| Marketing Start Date | 2015-08-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 63029-664-24 [63029066424]
GOODYS Extra Strength Headache Mixed Fruit Blast POWDER
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2015-08-01 |
| Inactivation Date | 2020-01-31 |
| Reactivation Date | 2020-04-10 |
NDC 63029-664-04 [63029066404]
GOODYS Extra Strength Headache Mixed Fruit Blast POWDER
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2015-08-01 |
| Inactivation Date | 2020-01-31 |
| Reactivation Date | 2020-04-10 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| CAFFEINE | 65 mg/1 |
OpenFDA Data
| SPL SET ID: | 6cb758bf-5a50-46bb-96e7-65b810b12de0 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| Pharm Class PE | |
| PHarm Class EPC | |
| NUI Code |
NDC Crossover Matching brand name "GOODYS Extra Strength Headache Mixed Fruit Blast" or generic name "Acetaminophen, Aspirin, And Caffeine"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 50066-286 | BioElectro Extra Strength | acetaminophen, aspirin, and caffeine |
| 66715-5738 | Circle K Headache Relief | Acetaminophen, Aspirin, and Caffeine |
| 0067-2040 | Excedrin | Acetaminophen, Aspirin, and Caffeine |
| 29485-8026 | EXCEDRIN EXTRA STRENGTH PAIN RELIEVER | ACETAMINOPHEN, ASPIRIN, and CAFFEINE |
| 29485-8027 | EXCEDRIN EXTRA STRENGTH PAIN RELIEVER | ACETAMINOPHEN, ASPIRIN, and CAFFEINE |
| 52904-871 | Excedrin Migraine | acetaminophen, aspirin, and caffeine |
| 29485-8028 | EXCEDRIN MIGRAINE acetaminophen, aspirin (nsaid) and caffeine | Acetaminophen, Aspirin, and Caffeine |
| 29485-0751 | Extra Strength Headach Relief | acetaminophen, aspirin, and caffeine |
| 0363-0021 | Extra Strength Headache Relief | Acetaminophen, Aspirin, and Caffeine |
| 29485-0752 | Extra Strength Headache Relief | acetaminophen, aspirin, and caffeine |
| 29485-6486 | Extra Strength Headache Relief | acetaminophen, aspirin, and caffeine |
| 55910-440 | Extra Strength Headache Relief | acetaminophen, aspirin, and caffeine |
| 63029-664 | GOODYS | acetaminophen, aspirin, and caffeine |
| 66715-5301 | Goodys Cool Orange | Acetaminophen, Aspirin, and Caffeine |
| 63029-625 | Goodys Extra Strength | Acetaminophen, Aspirin, and Caffeine |
| 63029-633 | Goodys Extra Strength | Acetaminophen, Aspirin, and Caffeine |
| 63029-644 | Goodys Extra Strength | Acetaminophen, Aspirin, and Caffeine |
| 63029-655 | Goodys Extra Strength | Acetaminophen, Aspirin, and Caffeine |
| 63029-662 | Goodys Extra Strength | Acetaminophen, Aspirin, and Caffeine |
| 63029-677 | Goodys Extra Strength | Acetaminophen, Aspirin, and Caffeine |
| 50405-008 | SohMed Pain Reliever | Acetaminophen, Aspirin, and Caffeine |
| 42364-0011 | vanquish | acetaminophen, aspirin, and caffeine |
| 59556-810 | vanquish | acetaminophen, aspirin, and caffeine |
| 42364-0122 | vanquish digital headache | acetaminophen, aspirin, and caffeine |
Trademark Results [GOODYS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GOODYS 90536996 not registered Live/Pending |
Dave Goodwin 2021-02-19 |
 GOODYS 76674028 not registered Dead/Abandoned |
Sarris, Minas 2007-03-12 |
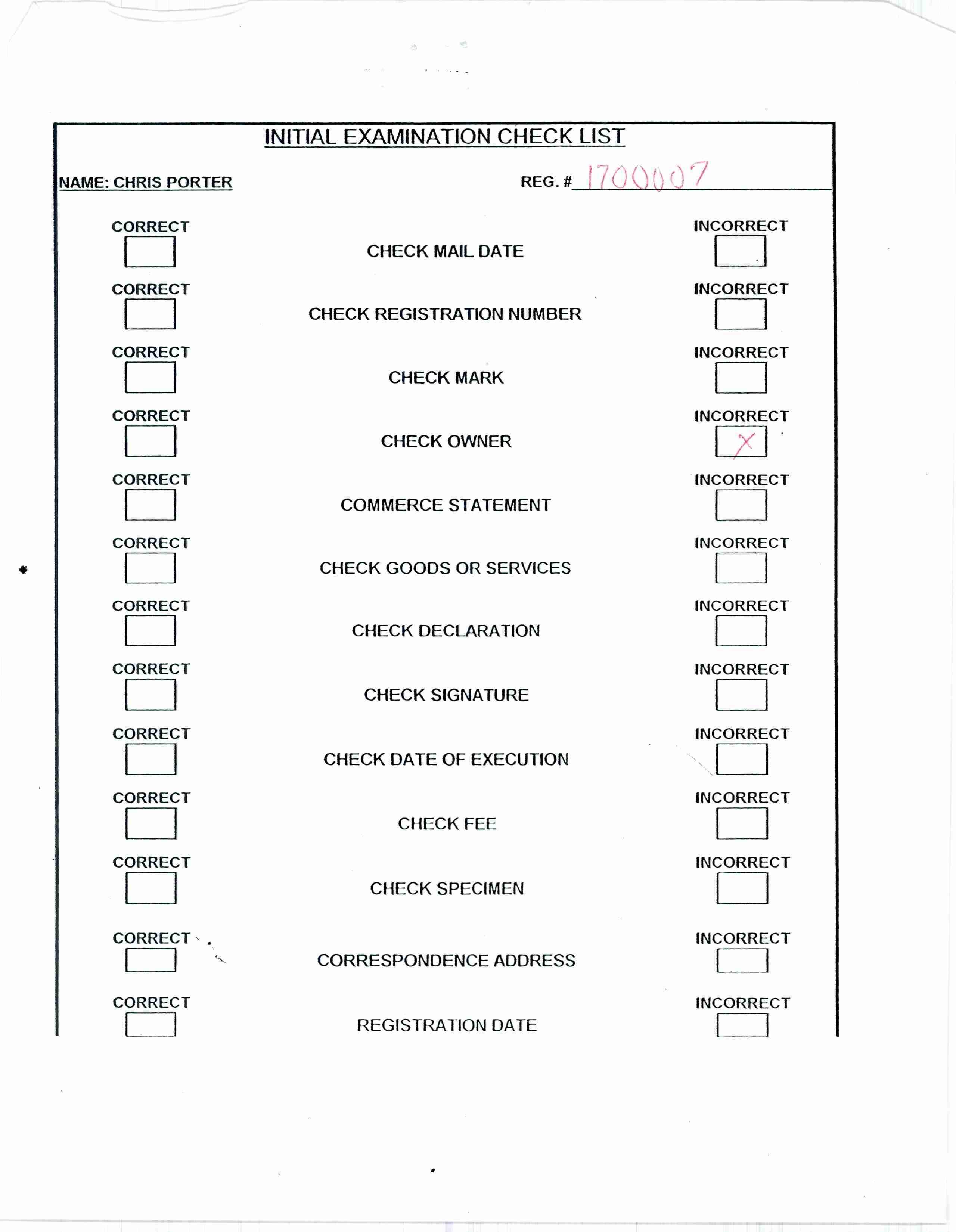 GOODYS 74110229 1700007 Dead/Cancelled |
INDUSTRIAL MATERIALS CORPORATION 1990-10-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.