NDC 63736-663
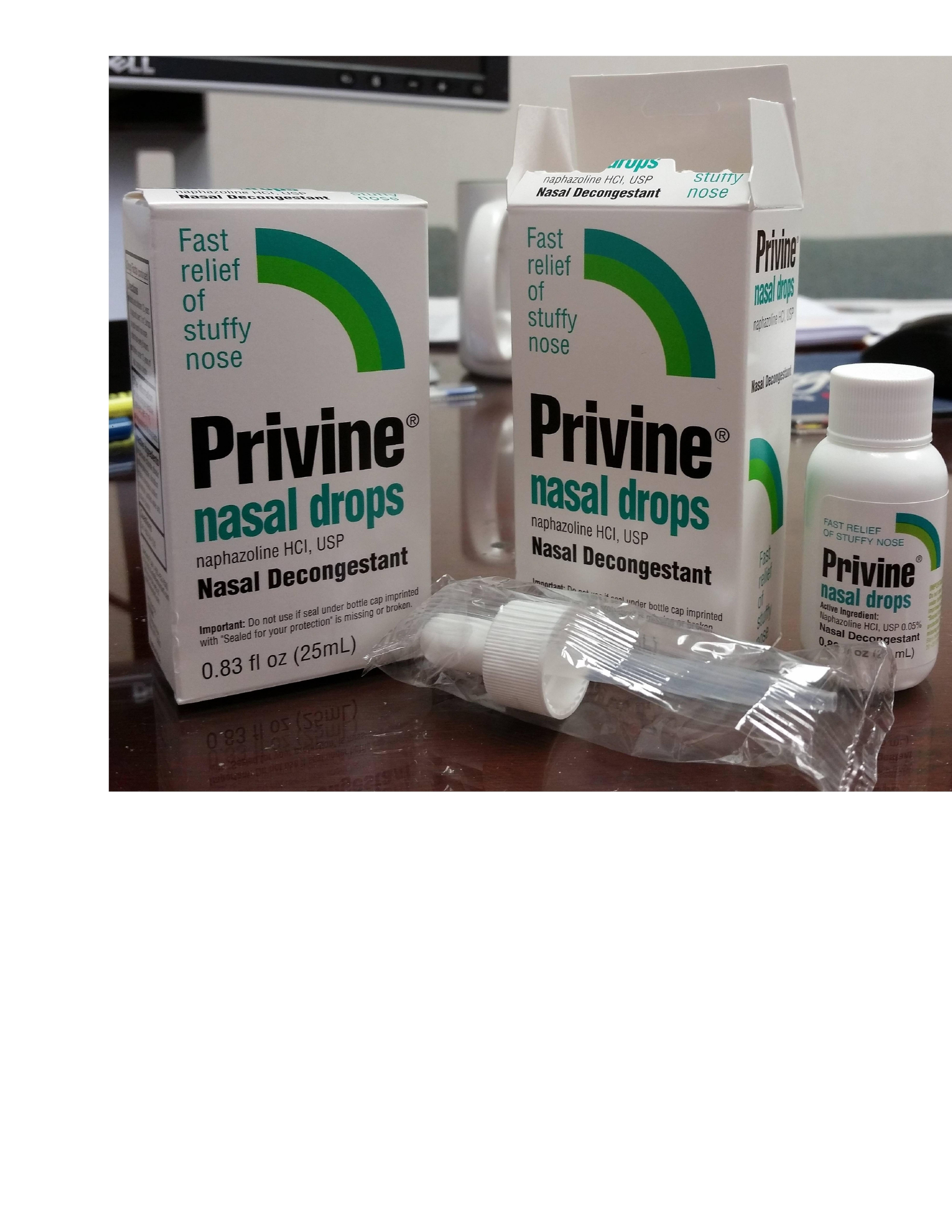
Privine
Naphazoline Hydrochloride
Privine is a Nasal Suspension/ Drops in the Human Otc Drug category. It is labeled and distributed by Insight Pharmaceuticals Llc. The primary component is Naphazoline Hydrochloride.
| Product ID | 63736-663_8541b271-ccd6-4c6d-9797-60335e7e4d22 |
| NDC | 63736-663 |
| Product Type | Human Otc Drug |
| Proprietary Name | Privine |
| Generic Name | Naphazoline Hydrochloride |
| Dosage Form | Suspension/ Drops |
| Route of Administration | NASAL |
| Marketing Start Date | 2009-07-16 |
| Marketing Category | OTC MONOGRAPH FINAL / OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Labeler Name | Insight Pharmaceuticals LLC |
| Substance Name | NAPHAZOLINE HYDROCHLORIDE |
| Active Ingredient Strength | 0 mL/25mL |
| NDC Exclude Flag | E |
| Listing Certified Through | 2017-12-31 |
Packaging
NDC 63736-663-01
1 BOTTLE in 1 BOX (63736-663-01) > 25 mL in 1 BOTTLE
| Marketing Start Date | 2009-07-16 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 63736-663-01 [63736066301]
Privine SUSPENSION/ DROPS
| Marketing Category | OTC monograph final |
| Application Number | part341 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2009-07-16 |
| Inactivation Date | 2019-10-29 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| NAPHAZOLINE HYDROCHLORIDE | .125 mL/25mL |
OpenFDA Data
| SPL SET ID: | ac1410ce-ae7f-498d-b84c-a6d32e1c87a6 |
| Manufacturer | |
| UNII |
NDC Crossover Matching brand name "Privine" or generic name "Naphazoline Hydrochloride"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 63736-662 | Privine | Naphazoline Hydrochloride |
| 63736-663 | Privine | Naphazoline Hydrochloride |
| 21695-915 | AK-Con | naphazoline hydrochloride |
| 16995-001 | FLORIL REDNESS RELIEF | NAPHAZOLINE HYDROCHLORIDE |
| 48462-001 | NAFASOLINA | NAPHAZOLINE HYDROCHLORIDE |
| 63187-412 | Naphazoline | Naphazoline Hydrochloride |
| 49873-057 | Nazal | Naphazoline hydrochloride |
| 51367-008 | Pi Yen Chin | Naphazoline Hydrochloride |
| 51367-035 | Pi Yen Chin | Naphazoline Hydrochloride |
| 49873-044 | Sato Clear | NAPHAZOLINE HYDROCHLORIDE |
Trademark Results [Privine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRIVINE 76221160 2546937 Live/Registered |
LEOSONS OVERSEAS CORPORATION 2001-03-06 |
 PRIVINE 71457000 0418496 Live/Registered |
CIBA PHARMACEUTICAL PRODUCTS, INC. 1942-11-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.