NDC 63742-001
Numb Master
Lidocaine
Numb Master is a Topical Cream in the Human Otc Drug category. It is labeled and distributed by Clinical Resolution Laboratory, Inc.. The primary component is Lidocaine.
| Product ID | 63742-001_2734a77a-249d-3780-e054-00144ff88e88 |
| NDC | 63742-001 |
| Product Type | Human Otc Drug |
| Proprietary Name | Numb Master |
| Generic Name | Lidocaine |
| Dosage Form | Cream |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2015-12-18 |
| Marketing Category | OTC MONOGRAPH FINAL / OTC MONOGRAPH FINAL |
| Application Number | part346 |
| Labeler Name | Clinical Resolution Laboratory, Inc. |
| Substance Name | LIDOCAINE |
| Active Ingredient Strength | 5 mg/g |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 63742-001-00
118 g in 1 BOTTLE (63742-001-00)
| Marketing Start Date | 2014-04-24 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 63742-001-00 [63742000100]
Numb Master CREAM
| Marketing Category | OTC monograph final |
| Application Number | part346 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2014-04-24 |
NDC 63742-001-01 [63742000101]
Numb Master CREAM
| Marketing Category | OTC monograph final |
| Application Number | part346 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2015-12-18 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| LIDOCAINE | 5 g/100g |
OpenFDA Data
| SPL SET ID: | 6d2428fc-4680-4dbd-9419-0374ea5a5060 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
NDC Crossover Matching brand name "Numb Master" or generic name "Lidocaine"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 63742-001 | Numb Master | Numb Master |
| 63742-031 | Numb Master | Numb Master |
| 0536-1267 | 4 Lidocaine Topical Anesthetic | LIDOCAINE |
| 0363-1114 | Anorectal | Lidocaine |
| 0113-7200 | basic care endure | lidocaine |
| 0113-7219 | basic care lidocaine | Lidocaine |
| 0113-7700 | basic care lidocaine | LIDOCAINE |
| 0363-9226 | First Aid Burn | Lidocaine |
| 0113-8000 | Good Sense endure | lidocaine |
| 0168-0204 | Lidocaine | Lidocaine |
| 0362-0221 | Lidocaine | Lidocaine |
| 0378-9055 | Lidocaine | lidocaine |
| 0440-9715 | Lidocaine | Lidocaine |
| 0404-9890 | Lidocaine Hydrochloride | Lidocaine |
| 0404-9893 | Lidocaine Hydrochloride | Lidocaine |
| 0496-0882 | LMX4 | lidocaine |
| 0496-0883 | LMX5 | lidocaine |
| 0363-3001 | Pain and Itch Relief | Lidocaine |
| 0363-9110 | PAIN RELIEF PATCHES | LIDOCAINE |
| 0363-9116 | PAIN RELIEF PATCHES | LIDOCAINE |
| 0496-0892 | RECTICARE | lidocaine |
| 0536-1139 | Rugby Lidocaine | Lidocaine |
| 0536-1278 | RUGBY LIDOCAINE | LIDOCAINE |
| 0536-1202 | Rugby PAIN RELIEF PATCHES | LIDOCAINE |
| 0363-4003 | Walgreens Aloe Vera | LIDOCAINE |
| 0363-0985 | Walgreens Sunburn Relief | Lidocaine |
Trademark Results [Numb Master]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
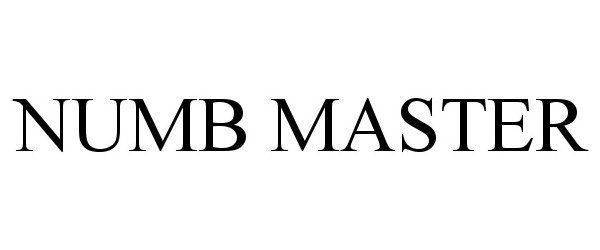 NUMB MASTER 97226054 not registered Live/Pending |
Lee, Justin 2022-01-18 |
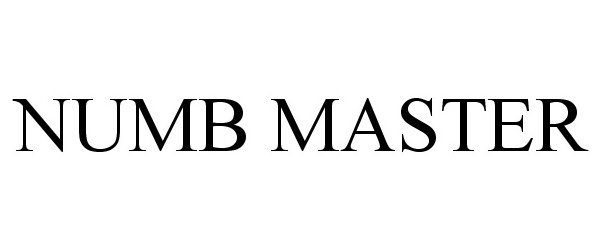 NUMB MASTER 86331253 4707268 Live/Registered |
Clinical Resolution Laboratory, Inc. 2014-07-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.