NDC 64616-072
Formula 3 Cough Syrup
Cough Syrup
Formula 3 Cough Syrup is a Oral Liquid in the Human Otc Drug category. It is labeled and distributed by Vitality Works, Inc.. The primary component is Pelargonium Sidoides Root; Prunus Serotina Bark; Sambucus Nigra Flower.
| Product ID | 64616-072_7c5eba5d-5e95-bf83-e053-2991aa0aa5e9 |
| NDC | 64616-072 |
| Product Type | Human Otc Drug |
| Proprietary Name | Formula 3 Cough Syrup |
| Generic Name | Cough Syrup |
| Dosage Form | Liquid |
| Route of Administration | ORAL |
| Marketing Start Date | 2010-07-07 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | Vitality Works, Inc. |
| Substance Name | PELARGONIUM SIDOIDES ROOT; PRUNUS SEROTINA BARK; SAMBUCUS NIGRA FLOWER |
| Active Ingredient Strength | 1 [hp_X]/mL; [hp_X]/mL; [hp_X]/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 64616-072-01
118 mL in 1 BOTTLE (64616-072-01)
| Marketing Start Date | 2010-07-07 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 64616-072-01 [64616007201]
Formula 3 Cough Syrup LIQUID
| Marketing Category | unapproved homeopathic |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2010-07-07 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| PELARGONIUM SIDOIDES ROOT | 1 [hp_X]/mL |
OpenFDA Data
| SPL SET ID: | 199bbe91-ebfe-45fa-acba-62b35c7e6fba |
| Manufacturer | |
| UNII | |
| UPC Code |
NDC Crossover Matching brand name "Formula 3 Cough Syrup" or generic name "Cough Syrup"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 64616-072 | Formula 3 Cough Syrup | Cough Syrup |
| 62106-1181 | Drosera Plex | Cough Syrup |
| 62106-3126 | Drosera Plex | Cough Syrup |
Trademark Results [Formula 3 Cough Syrup]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
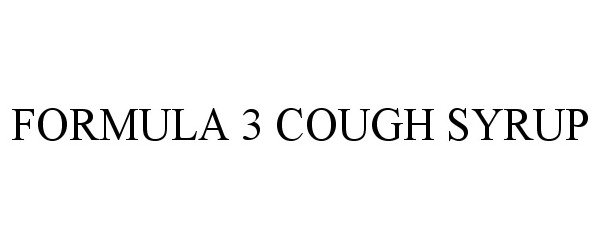 FORMULA 3 COUGH SYRUP 85362915 4111426 Live/Registered |
Clarke, Murray 2011-07-05 |
 FORMULA 3 COUGH SYRUP 85275716 not registered Dead/Abandoned |
Murray Clarke 2011-03-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.