NDC 64616-082
Category I
Immune System Booster
Category I is a Oral Liquid in the Human Otc Drug category. It is labeled and distributed by Vitality Works, Inc. The primary component is Actaea Spicata Root; Anagallis Arvensis; Argemone Mexicana; Candida Albicans; Heloderma Horridum Venom; Hyoscyamus Niger; Potassium Dichromate; Potassium Iodide; Potassium Nitrate; Potassium Phosphate, Dibasic; Senna Leaf; Spongia Officinalis Skeleton, Roasted.
| Product ID | 64616-082_615773bc-088d-fc39-e053-2a91aa0af00f |
| NDC | 64616-082 |
| Product Type | Human Otc Drug |
| Proprietary Name | Category I |
| Generic Name | Immune System Booster |
| Dosage Form | Liquid |
| Route of Administration | ORAL |
| Marketing Start Date | 2013-03-20 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | Vitality Works, Inc |
| Substance Name | ACTAEA SPICATA ROOT; ANAGALLIS ARVENSIS; ARGEMONE MEXICANA; CANDIDA ALBICANS; HELODERMA HORRIDUM VENOM; HYOSCYAMUS NIGER; POTASSIUM DICHROMATE; POTASSIUM IODIDE; POTASSIUM NITRATE; POTASSIUM PHOSPHATE, DIBASIC; SENNA LEAF; SPONGIA OFFICINALIS SKELETON, ROASTED |
| Active Ingredient Strength | 6 [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 64616-082-02
59 mL in 1 BOTTLE, DROPPER (64616-082-02)
| Marketing Start Date | 2013-04-16 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 64616-082-02 [64616008202]
Category I LIQUID
| Marketing Category | unapproved homeopathic |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2013-04-16 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ACTAEA SPICATA ROOT | 6 [hp_X]/mL |
OpenFDA Data
| SPL SET ID: | 1cbb330b-b541-457b-9a97-e17a5837d30d |
| Manufacturer | |
| UNII |
NDC Crossover Matching brand name "Category I" or generic name "Immune System Booster"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 64616-082 | Category I | Immune System Booster |
| 64616-083 | Category II | Immune System Booster |
| 64616-084 | Category III | Immune System Booster |
| 64616-085 | Category IV | Immune System Booster |
| 64616-086 | Category V | Immune System Booster |
Trademark Results [Category I]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
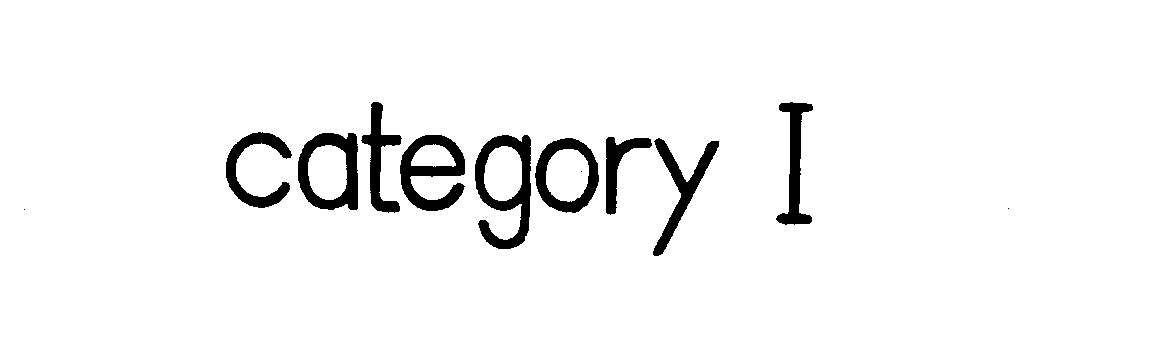 CATEGORY I 73151407 1117320 Dead/Cancelled |
A.F.A. KNITS, INC. 1977-12-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.