NDC 64942-1245
St. Ives Blemish and Blackhead Control Apricot Scrub
Salicylic Acid
St. Ives Blemish and Blackhead Control Apricot Scrub is a Topical Emulsion in the Human Otc Drug category. It is labeled and distributed by Conopco Inc. D/b/a Unilever. The primary component is Salicylic Acid.
| Product ID | 64942-1245_5bccc639-0fc5-48e7-b3b3-9e1ed536e878 |
| NDC | 64942-1245 |
| Product Type | Human Otc Drug |
| Proprietary Name | St. Ives Blemish and Blackhead Control Apricot Scrub |
| Generic Name | Salicylic Acid |
| Dosage Form | Emulsion |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2012-03-01 |
| Marketing Category | OTC MONOGRAPH FINAL / OTC MONOGRAPH FINAL |
| Application Number | part333D |
| Labeler Name | CONOPCO Inc. d/b/a Unilever |
| Substance Name | SALICYLIC ACID |
| Active Ingredient Strength | 0 g/g |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 64942-1245-2
212 g in 1 TUBE (64942-1245-2)
| Marketing Start Date | 2012-03-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 64942-1245-2 [64942124502]
St. Ives Blemish and Blackhead Control Apricot Scrub EMULSION
| Marketing Category | OTC monograph final |
| Application Number | part333D |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-03-01 |
| Inactivation Date | 2020-01-31 |
NDC 64942-1245-3 [64942124503]
St. Ives Blemish and Blackhead Control Apricot Scrub EMULSION
| Marketing Category | OTC monograph final |
| Application Number | part333D |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-03-01 |
| Inactivation Date | 2020-01-31 |
NDC 64942-1245-1 [64942124501]
St. Ives Blemish and Blackhead Control Apricot Scrub EMULSION
| Marketing Category | OTC monograph final |
| Application Number | part333D |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-03-01 |
| Inactivation Date | 2020-01-31 |
NDC 64942-1245-4 [64942124504]
St. Ives Blemish and Blackhead Control Apricot Scrub EMULSION
| Marketing Category | OTC monograph final |
| Application Number | part333D |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-03-01 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| SALICYLIC ACID | .02 g/g |
OpenFDA Data
| SPL SET ID: | d1863437-02cb-4766-9c2d-2d64367d4cee |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
NDC Crossover Matching brand name "St. Ives Blemish and Blackhead Control Apricot Scrub" or generic name "Salicylic Acid"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 64942-1245 | St. Ives Blemish and Blackhead Control Apricot Scrub | St. Ives Blemish and Blackhead Control Apricot Scrub |
| 64942-1860 | St. Ives Blemish and Blackhead Control Apricot Scrub | St. Ives Blemish and Blackhead Control Apricot Scrub |
| 64942-1859 | St. Ives Blemish and Blackhead Control Apricot Scrub | St. Ives Blemish and Blackhead Control Apricot Scrub |
| 0187-1638 | AcneFree | salicylic acid |
| 0187-1639 | AcneFree | salicylic acid |
| 0187-5010 | AcneFree Body Clearing | Salicylic Acid |
| 0311-0717 | Almay Clear Complexion 4 in 1 Pressed Powder | Salicylic Acid |
| 0311-0722 | Almay Clear Complexion Blemish Armor | Salicylic Acid |
| 0311-0716 | Almay Clear Complexion Blemish Healing Spot Concealer | Salicylic Acid |
| 0311-0718 | Almay Clear Complexion Blemish Healing Spot Concealer | Salicylic Acid |
| 0311-0725 | ALMAY CLEAR COMPLEXION CONCEALER | SALICYLIC ACID |
| 0311-0720 | Almay Clear Complexion Liquid Makeup | Salicylic Acid |
| 0311-0715 | Almay Clear Complexion Liquid Makeup for Oily Skin | Salicylic Acid |
| 0311-0724 | ALMAY CLEAR COMPLEXION MAKEUP | SALICYLIC ACID |
| 0311-0721 | Almay Clear Complexion Pressed Powder | Salicylic Acid |
| 0187-5471 | Ambi Even and Clear Exfoliating Wash | Salicylic Acid |
| 0187-5472 | Ambi Even and Clear Foaming Cleanser | Salicylic Acid |
| 0299-3674 | Benzac | salicylic acid |
| 0299-3676 | Benzac | salicylic acid |
| 0299-3677 | Benzac | salicylic acid |
| 0299-4121 | Cetaphil Acne Relief Body Wash | Salicylic Acid |
| 0299-4119 | Cetaphil Gentle Clear Clarifying Acne Cleanser | Salicylic Acid |
| 0299-4117 | Cetaphil Gentle Clear Mattifying Acne Moisturizer | Salicylic Acid |
| 0299-4135 | Cetaphil Gentle Clear Triple-Action Acne Serum | Salicylic Acid |
| 0187-6100 | CLENZIDERM DAILY CARE FOAMING CLEANSER | SALICYLIC ACID |
| 0187-6102 | CLENZIDERM PORE THERAPY | SALICYLIC ACID |
| 0299-4132 | Differin Acne Clearing Body | Salicylic Acid |
| 0299-4609 | Differin Acne Clearing Body Scrub | Salicylic Acid |
| 0299-4131 | Differin Acne Clearing Body Wash | Salicylic Acid |
| 0295-1039 | Salex | Salicylic acid |
| 0295-1042 | Salex | Salicylic acid |
| 0295-1040 | Salex Cream | Salicylic acid |
| 0363-0017 | Salicylic acid | Salicylic acid |
| 0220-4526 | Salicylicum acidum | SALICYLIC ACID |
| 0220-4528 | Salicylicum acidum | SALICYLIC ACID |
| 0023-4948 | SKIN MEDICA Purifying Foaming Wash | salicylic acid |
| 0295-0071 | Zapzyt Acne Wash Cleanser | Salicylic Acid |
| 0295-9055 | Zapzyt Acne Wash Cleanser | Salicylic Acid |
| 0295-1076 | Zapzyt Pore Cleaning Scrub | Salicylic Acid |
Trademark Results [St. Ives]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ST. IVES 85619563 4429533 Live/Registered |
ST. IVES LABORATORIES, INC. 2012-05-08 |
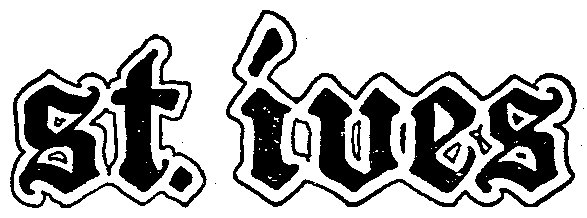 ST. IVES 81044393 1044393 Dead/Cancelled |
Tinder Box International, Ltd., The 0000-00-00 |
 ST. IVES 77536086 3586632 Live/Registered |
MCC Mecosta LLC 2008-07-31 |
 ST. IVES 74500481 1883622 Dead/Cancelled |
Simmons Company 1994-03-10 |
 ST. IVES 74304736 not registered Dead/Abandoned |
I.D. Enterprises, Inc. 1992-08-17 |
 ST. IVES 74274824 1818331 Dead/Cancelled |
St. Ives Laboratories, Inc. 1992-05-12 |
 ST. IVES 74241740 1902103 Live/Registered |
St. Ives Laboratories, Inc. 1992-01-30 |
 ST. IVES 74232187 not registered Dead/Abandoned |
National Wine & Spirits Corporation 1991-12-19 |
 ST. IVES 74088675 not registered Dead/Abandoned |
St. Ives Laboratories, Inc. 1990-08-16 |
 ST. IVES 73548887 1386479 Live/Registered |
ST. IVES LABORATORIES, INC. 1985-07-19 |
 ST. IVES 73390966 1307036 Dead/Cancelled |
Dimensions Unlimited, Inc. 1982-09-28 |
 ST. IVES 73253539 1164415 Dead/Cancelled |
General Mills, Inc. 1980-03-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.