NDC 67751-168
Tylenol PM
Acetaminophen, Diphenhydramine Hydrochloride
Tylenol PM is a Oral Tablet in the Human Otc Drug category. It is labeled and distributed by Navajo Manufacturing Company Inc.. The primary component is Acetaminophen; Diphenhydramine Hydrochloride.
| Product ID | 67751-168_3d1f84be-fde9-1789-e054-00144ff88e88 |
| NDC | 67751-168 |
| Product Type | Human Otc Drug |
| Proprietary Name | Tylenol PM |
| Generic Name | Acetaminophen, Diphenhydramine Hydrochloride |
| Dosage Form | Tablet |
| Route of Administration | ORAL |
| Marketing Start Date | 2016-09-22 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Labeler Name | Navajo Manufacturing Company Inc. |
| Substance Name | ACETAMINOPHEN; DIPHENHYDRAMINE HYDROCHLORIDE |
| Active Ingredient Strength | 500 mg/1; mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 67751-168-01
1 POUCH in 1 CARTON (67751-168-01) > 2 TABLET, FILM COATED in 1 POUCH
| Marketing Start Date | 2016-09-22 |
| Marketing End Date | 2021-05-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 67751-168-01 [67751016801]
Tylenol PM TABLET, FILM COATED
| Marketing Category | OTC monograph not final |
| Application Number | part343 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2016-09-22 |
| Marketing End Date | 2021-05-01 |
NDC 67751-168-02 [67751016802]
Tylenol PM TABLET, FILM COATED
| Marketing Category | OTC monograph not final |
| Application Number | part343 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2016-09-22 |
| Marketing End Date | 2021-05-01 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ACETAMINOPHEN | 500 mg/1 |
OpenFDA Data
| SPL SET ID: | a729cb7c-2845-4f37-85a1-97e459686e4d |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
NDC Crossover Matching brand name "Tylenol PM" or generic name "Acetaminophen, Diphenhydramine Hydrochloride"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 50269-608 | Tylenol PM | ACETAMINOPHEN, DIPHENHYDRAMINE HYDROCHLORIDE |
| 50580-608 | Tylenol PM | acetaminophen and diphenhydramine hydrochloride |
| 50580-833 | TYLENOL PM | Acetaminophen and Diphenhydramine Hydrochloride |
| 52904-945 | Tylenol PM | acetaminophen and diphenhydramine hydrochloride |
| 67414-608 | Tylenol PM | ACETAMINOPHEN, DIPHENHYDRAMINE HYDROCHLORIDE |
| 67751-168 | Tylenol PM | Tylenol PM |
| 73097-013 | Tylenol PM | Tylenol PM |
| 0536-1173 | Acetaminophen, Diphenhydramine Hydrochloride | Acetaminophen, Diphenhydramine Hydrochloride |
| 0904-6731 | Acetaminophen, Diphenhydramine Hydrochloride | Acetaminophen, Diphenhydramine Hydrochloride |
| 58602-216 | Aurophen Pain Away plus Sleep aid | acetaminophen, diphenhydramine hydrochloride |
| 58602-217 | Aurophen Pain Away plus Sleep aid | acetaminophen, diphenhydramine hydrochloride |
| 62011-0024 | Extra Strength Acetaminophen PM | ACETAMINOPHEN, DIPHENHYDRAMINE HYDROCHLORIDE |
| 0904-7651 | Extra Strength Mapap PM | ACETAMINOPHEN, DIPHENHYDRAMINE HYDROCHLORIDE |
| 59726-360 | Extra Strength Pain Relief PM | ACETAMINOPHEN, DIPHENHYDRAMINE HYDROCHLORIDE |
| 0363-8590 | NightTime Pain Reliever PM | acetaminophen, diphenhydramine hydrochloride |
| 10202-946 | Pain Away plus Sleep aid | acetaminophen, diphenhydramine hydrochloride |
| 46122-358 | Pain Plus Sleep | acetaminophen, diphenhydramine hydrochloride |
| 55910-996 | Pain Relief plus Sleep Aid | acetaminophen, diphenhydramine hydrochloride |
| 53943-028 | Pain Relief PM | acetaminophen, diphenhydramine hydrochloride |
| 55319-998 | Pain Relief PM | acetaminophen, diphenhydramine hydrochloride |
| 59779-905 | Pain Relief PM | acetaminophen, diphenhydramine hydrochloride |
| 54257-278 | Pain Reliever PM | ACETAMINOPHEN, DIPHENHYDRAMINE HYDROCHLORIDE |
Trademark Results [Tylenol PM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
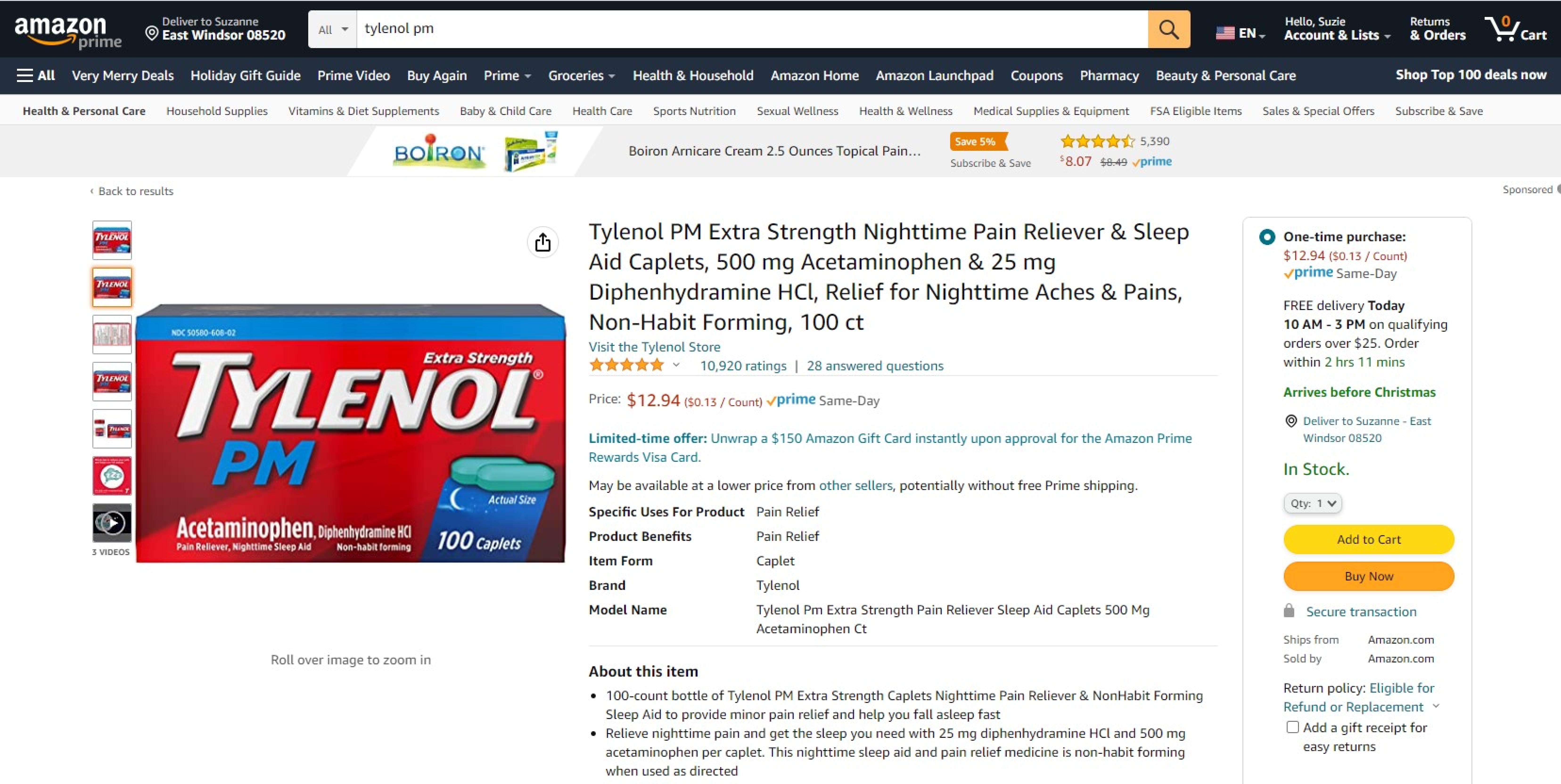 TYLENOL PM 74143985 1777613 Live/Registered |
JOHNSON & JOHNSON 1991-03-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.