NDC 68084-572
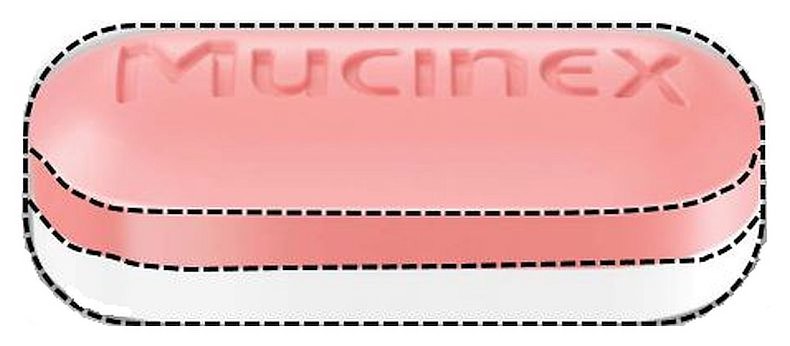
Mucinex
Guaifenesin
Mucinex is a Oral Tablet, Extended Release in the Human Otc Drug category. It is labeled and distributed by American Health Packaging. The primary component is Guaifenesin.
| Product ID | 68084-572_826c9e76-700d-f017-e053-2a91aa0aba13 |
| NDC | 68084-572 |
| Product Type | Human Otc Drug |
| Proprietary Name | Mucinex |
| Generic Name | Guaifenesin |
| Dosage Form | Tablet, Extended Release |
| Route of Administration | ORAL |
| Marketing Start Date | 2016-02-10 |
| Marketing Category | NDA / NDA |
| Application Number | NDA021282 |
| Labeler Name | American Health Packaging |
| Substance Name | GUAIFENESIN |
| Active Ingredient Strength | 600 mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 68084-572-01
100 BLISTER PACK in 1 BOX, UNIT-DOSE (68084-572-01) > 1 TABLET, EXTENDED RELEASE in 1 BLISTER PACK (68084-572-11)
| Marketing Start Date | 2016-02-10 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 68084-572-01 [68084057201]
Mucinex TABLET, EXTENDED RELEASE
| Marketing Category | NDA |
| Application Number | NDA021282 |
| Product Type | HUMAN OTC DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2016-02-10 |
NDC 68084-572-11 [68084057211]
Mucinex TABLET, EXTENDED RELEASE
| Marketing Category | NDA |
| Application Number | NDA021282 |
| Product Type | HUMAN OTC DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2016-02-10 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| GUAIFENESIN | 600 mg/1 |
OpenFDA Data
| SPL SET ID: | 0129f47d-abc2-414c-b4e1-1064c5d6a623 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Medicade Reported Pricing
68084057211 MUCINEX ER 600 MG TABLET
Pricing Unit: EA | Drug Type:
68084057201 MUCINEX ER 600 MG TABLET
Pricing Unit: EA | Drug Type:
NDC Crossover Matching brand name "Mucinex" or generic name "Guaifenesin"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 68084-572 | Mucinex | Mucinex |
| 70518-0228 | Mucinex | Mucinex |
| 70518-0466 | Mucinex | Mucinex |
| 17856-0008 | Mucinex | Mucinex |
| 21695-870 | Mucinex | Mucinex |
| 50090-0818 | Mucinex | Mucinex |
| 50090-1340 | Mucinex | Mucinex |
| 50090-2380 | Mucinex | Mucinex |
| 53808-0871 | Mucinex | Mucinex |
| 55045-3143 | MUCINEX | MUCINEX |
| 55154-4680 | Mucinex | Mucinex |
| 55700-489 | Mucinex | Mucinex |
| 61919-651 | MUCINEX | MUCINEX |
| 63187-954 | Mucinex | Mucinex |
| 63629-7115 | Mucinex | Mucinex |
| 63629-7886 | Mucinex | Mucinex |
| 63824-008 | Mucinex | Mucinex |
| 67544-149 | Mucinex | Mucinex |
| 0363-0288 | childrens chest congestion | Guaifenesin |
| 0113-0325 | Good Sense Mucus ER | Guaifenesin |
| 0113-2023 | good sense mucus er | guaifenesin |
| 0113-3650 | Good Sense Mucus ER | Guaifenesin |
| 0113-0061 | good sense tussin | Guaifenesin |
| 0121-0744 | GUAIFENESIN | GUAIFENESIN |
| 0121-1488 | GUAIFENESIN | GUAIFENESIN |
| 0121-1744 | GUAIFENESIN | GUAIFENESIN |
| 0121-2232 | GUAIFENESIN | GUAIFENESIN |
| 0363-0028 | Guaifenesin | Guaifenesin |
| 0363-0033 | Guaifenesin | Guaifenesin |
| 0363-0071 | Mucus | Guaifenesin |
| 0363-0074 | Mucus | Guaifenesin |
| 0363-0532 | Mucus Relief | Guaifenesin |
| 0363-0325 | mucus relief er | Guaifenesin |
| 0031-9303 | Robitussin Direct Chest Congestion | guaifenesin |
| 0031-8724 | Robitussin Mucus Plus Chest Congestion | Guaifenesin |
| 0113-2061 | tussin | Guaifenesin |
| 0363-0310 | wal tussin | Guaifenesin |
Trademark Results [Mucinex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MUCINEX 98499792 not registered Live/Pending |
RB Health (US) LLC 2024-04-15 |
 MUCINEX 90229382 not registered Live/Pending |
RB Health (US) LLC 2020-10-01 |
 MUCINEX 88040437 not registered Live/Pending |
Reckitt Benckiser LLC 2018-07-17 |
 MUCINEX 86191608 4613098 Live/Registered |
RB HEALTH (US) LLC 2014-02-12 |
 MUCINEX 85496330 4168070 Live/Registered |
RB HEALTH (US) LLC 2011-12-15 |
 MUCINEX 85489681 4168052 Live/Registered |
RB HEALTH (US) LLC 2011-12-07 |
 MUCINEX 77770135 3722363 Live/Registered |
Reckitt Benckiser Inc. 2009-06-29 |
 MUCINEX 76297961 2670161 Live/Registered |
RB HEALTH (US) LLC 2001-08-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.