NDC 68828-089
Cocoa Always Color Stay-On Makeup SPF 10
Titanium Dioxide, Octinoxate/ethylhexylmehtoxycinnamate
Cocoa Always Color Stay-On Makeup SPF 10 is a Topical Lotion in the Human Otc Drug category. It is labeled and distributed by Jafra Cosmetics International Inc. The primary component is Titanium Dioxide; Octinoxate.
| Product ID | 68828-089_538de5c6-e757-42b9-b7eb-0a0f5da40ba5 |
| NDC | 68828-089 |
| Product Type | Human Otc Drug |
| Proprietary Name | Cocoa Always Color Stay-On Makeup SPF 10 |
| Generic Name | Titanium Dioxide, Octinoxate/ethylhexylmehtoxycinnamate |
| Dosage Form | Lotion |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2012-05-16 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / OTC MONOGRAPH NOT FINAL |
| Application Number | part352 |
| Labeler Name | Jafra Cosmetics International Inc |
| Substance Name | TITANIUM DIOXIDE; OCTINOXATE |
| Active Ingredient Strength | 8 g/100mL; g/100mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 68828-089-01
1 BOTTLE, GLASS in 1 BOX (68828-089-01) > 30 mL in 1 BOTTLE, GLASS
| Marketing Start Date | 2012-05-16 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 68828-089-01 [68828008901]
Cocoa Always Color Stay-On Makeup SPF 10 LOTION
| Marketing Category | OTC monograph not final |
| Application Number | part352 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-05-16 |
NDC 68828-089-02 [68828008902]
Cocoa Always Color Stay-On Makeup SPF 10 LOTION
| Marketing Category | OTC monograph not final |
| Application Number | part352 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2019-06-07 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| TITANIUM DIOXIDE | 8 mL/100mL |
OpenFDA Data
| SPL SET ID: | c41813a8-bcf3-469e-8a7c-a8fe13d72ea1 |
| Manufacturer | |
| UNII |
NDC Crossover Matching brand name "Cocoa Always Color Stay-On Makeup SPF 10" or generic name "Titanium Dioxide, Octinoxate/ethylhexylmehtoxycinnamate"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 68828-083 | Cafe Au Lait | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-088 | Cafe Mocha | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-081 | Cashmere | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-089 | Cocoa | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-080 | Desert Beige | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-086 | Golden Tan | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-076 | Ivory | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-087 | Natural Tan | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-077 | Quiet Rose | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-079 | Sandy Beige | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-078 | Simply Beige | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-090 | Soft Beige | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-082 | Toast | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-085 | Toffee | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
| 68828-084 | Warm Ginger | Titanium Dioxide, Octinoxate/EthylhexylMehtoxycinnamate |
Trademark Results [Cocoa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
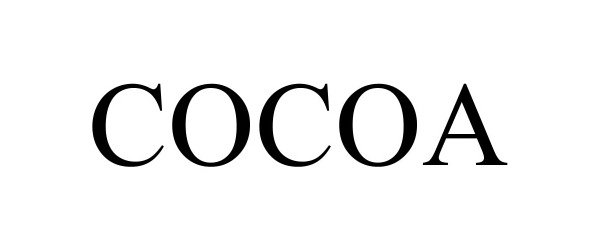 COCOA 98148467 not registered Live/Pending |
COCOA ADVISOR LIMITED 2023-08-24 |
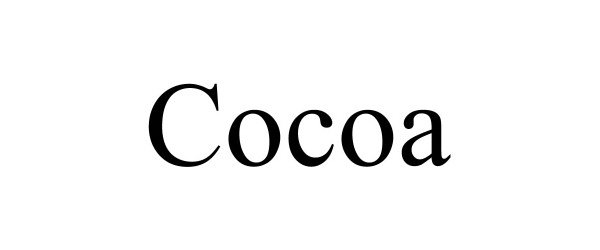 COCOA 90390200 not registered Live/Pending |
Maison Battat Inc. 2020-12-17 |
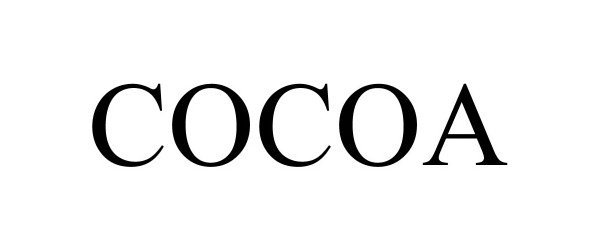 COCOA 90361662 not registered Live/Pending |
Phillips-Boyd, Serlina 2020-12-06 |
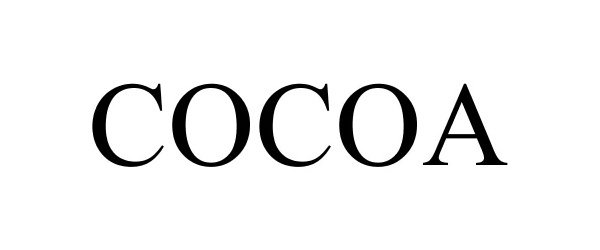 COCOA 87931677 not registered Dead/Abandoned |
Espro, Inc. 2018-05-22 |
 COCOA 87189724 not registered Dead/Abandoned |
Maison Joseph Battat, Ltd./Ltee. 2016-09-30 |
 COCOA 86001194 not registered Dead/Abandoned |
Cocoa Corporation 2013-07-02 |
 COCOA 86001192 not registered Dead/Abandoned |
Cocoa Corporation 2013-07-02 |
 COCOA 85133664 not registered Dead/Abandoned |
LUV TRADING, INC. 2010-09-20 |
 COCOA 78850364 not registered Dead/Abandoned |
Ty Inc. 2006-03-30 |
 COCOA 78259695 not registered Dead/Abandoned |
COCOA CIGAR INCORPORATED 2003-06-08 |
 COCOA 77343934 not registered Dead/Abandoned |
WARNER, SCOTT 2007-12-04 |
 COCOA 77147136 3587807 Dead/Cancelled |
WARNER, SCOTT 2007-04-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.