NDC 69336-202
Flexipak
Diclofenac Sodium, Capsaicin
Flexipak is a Kit in the Human Prescription Drug category. It is labeled and distributed by Sterling-knight Pharmaceuticals, Llc. The primary component is .
| Product ID | 69336-202_74e88119-d95e-4e1c-aa98-ebf8a8b91a92 |
| NDC | 69336-202 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Flexipak |
| Generic Name | Diclofenac Sodium, Capsaicin |
| Dosage Form | Kit |
| Marketing Start Date | 2018-01-19 |
| Marketing Category | ANDA / ANDA |
| Application Number | ANDA075185 |
| Labeler Name | Sterling-Knight Pharmaceuticals, LLC |
| Active Ingredient Strength | 0 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 69336-202-01
1 KIT in 1 CARTON (69336-202-01) * 60 TABLET, DELAYED RELEASE in 1 BOTTLE (61442-103-60) * 1 TUBE in 1 CARTON (0536-2525-25) > 60 g in 1 TUBE
| Marketing Start Date | 2018-01-19 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 69336-202-01 [69336020201]
Flexipak KIT
| Marketing Category | ANDA |
| Application Number | ANDA075185 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2018-01-19 |
| Inactivation Date | 2020-01-31 |
Drug Details
OpenFDA Data
| SPL SET ID: | 7c14a409-88c5-4b9d-80a4-f9f80c64ebbf |
| Manufacturer | |
| RxNorm Concept Unique ID - RxCUI |
NDC Crossover Matching brand name "Flexipak" or generic name "Diclofenac Sodium, Capsaicin"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 69336-202 | Flexipak | diclofenac sodium, capsaicin |
| 59088-343 | DermacinRx Lexitral PharmaPak | diclofenac sodium, capsaicin |
| 59088-784 | DermacinRx Lexitral PharmaPak II | diclofenac sodium, capsaicin |
| 81877-502 | DicloHeal-60 | diclofenac sodium, capsaicin |
| 69336-203 | Diclopak | diclofenac sodium, capsaicin |
| 69329-510 | Diclotral Pak | diclofenac sodium, capsaicin |
| 72275-731 | Inavix | Diclofenac Sodium, Capsaicin |
| 59088-093 | Inflammacin | diclofenac sodium, capsaicin |
| 70859-003 | NuDiclo SoluPak | diclofenac sodium, capsaicin |
| 70859-004 | NuDiclo TabPak | DICLOFENAC SODIUM, Capsaicin |
| 69837-500 | Sure Result DSS Premium Pak | DICLOFENAC SODIUM, CAPSAICIN |
| 69621-343 | Xelitral | diclofenac sodium, capsaicin |
| 69621-093 | Xenaflamm | diclofenac sodium, capsaicin |
Trademark Results [Flexipak]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
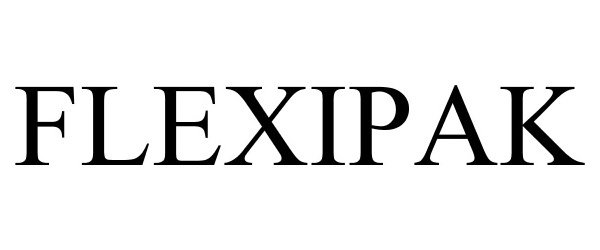 FLEXIPAK 87017812 5113332 Live/Registered |
Master Bond, Inc. 2016-04-28 |
 FLEXIPAK 76065838 2533741 Dead/Cancelled |
GE HEALTHCARE AS 2000-06-09 |
 FLEXIPAK 75713185 2628633 Dead/Cancelled |
FLEXIPAK TAPE PRODUCTS, L.L.C. 1999-05-24 |
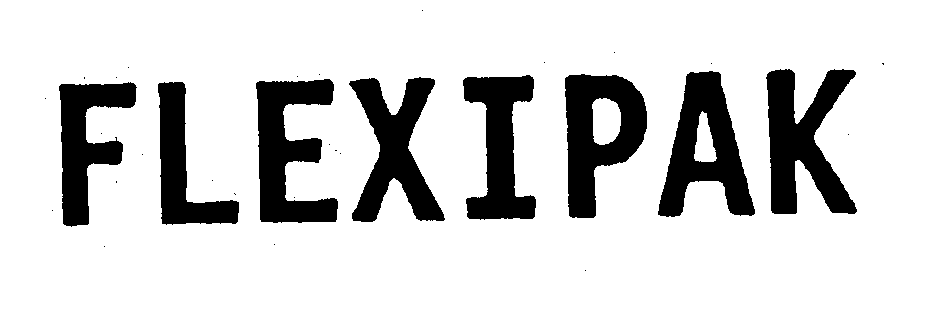 FLEXIPAK 74193989 1895569 Dead/Cancelled |
STERLING WINTHROP INC. 1991-08-12 |
 FLEXIPAK 73365335 1263469 Dead/Cancelled |
K. L. Rubel and Associates, Ltd. 1982-05-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.