NDC 70327-0002
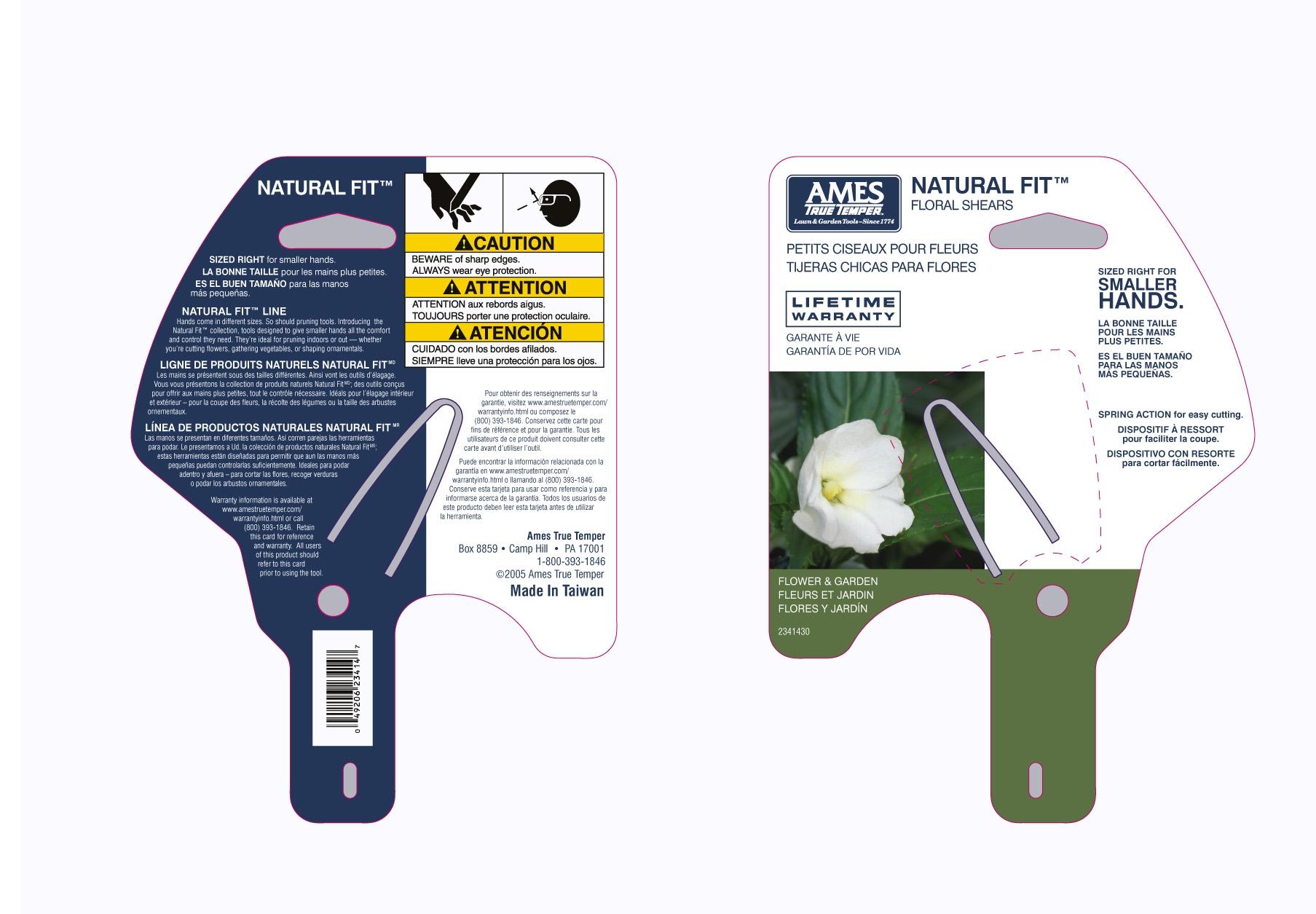
Natural Fit
Kali Phosphoricum, Natrum Sulphuricum, Agnus Castus, Chelidonium Majus, Fucus Vesiculosus, Lycopodium Clavatum, Thuja Occidentalis, Capsicum Annuum, Carduus Marianus, Cysteinum, Hydrocotyle Asiatica, Kali Carbonicum, Nux Vomica, Phytolacca Decandra, Rosa Canina, Flos, Salix Alba, Thea Sinensis, Gambogia, Carbo Vegetabilis, Calcarea Carbonica, Graphites, Natrum Muriaticum, Natrum Phosphoricum, Glandula Suprarenalis Suis, Hepar Suis, Pancreas Suis, Pituitarum Posterium (bovine), Placenta Totalis Suis,
Natural Fit is a Oral Liquid in the Human Otc Drug category. It is labeled and distributed by Advanced Healthcare Solutions. The primary component is Dibasic Potassium Phosphate; Sodium Sulfate; Chaste Tree Fruit; Chelidonium Majus Whole; Fucus Vesiculosus; Lycopodium Clavatum Spore; Thuja Occidentalis Leafy Twig; Capsicum; Milk Thistle; Cysteine; Centella Asiatica Whole; Potassium Carbonate; Strychnos Nux-vomica Seed; Phytolacca Americana Root; Rosa Canina Flower; Salix Alba Bark; Green Tea Leaf; Gamboge; Activated Charcoal; Oyster Shell Calcium Carbonate, Crude; Graphite; Sodium Chloride; Sodium Phosphate, Dibasic, Heptahydrate; Sus Scrofa Adrenal Gland; Pork Liver; Sus Scrofa Pancreas; Bos Taurus Pituitary Gland, Posterior; Sus Scrofa Placenta; Thyroid, Bovine; Gymnema Sylvestre Leaf; Water.
| Product ID | 70327-0002_87b4b9d3-1f5e-4d55-9385-9d60ff271503 |
| NDC | 70327-0002 |
| Product Type | Human Otc Drug |
| Proprietary Name | Natural Fit |
| Generic Name | Kali Phosphoricum, Natrum Sulphuricum, Agnus Castus, Chelidonium Majus, Fucus Vesiculosus, Lycopodium Clavatum, Thuja Occidentalis, Capsicum Annuum, Carduus Marianus, Cysteinum, Hydrocotyle Asiatica, Kali Carbonicum, Nux Vomica, Phytolacca Decandra, Rosa Canina, Flos, Salix Alba, Thea Sinensis, Gambogia, Carbo Vegetabilis, Calcarea Carbonica, Graphites, Natrum Muriaticum, Natrum Phosphoricum, Glandula Suprarenalis Suis, Hepar Suis, Pancreas Suis, Pituitarum Posterium (bovine), Placenta Totalis Suis, |
| Dosage Form | Liquid |
| Route of Administration | ORAL |
| Marketing Start Date | 2020-08-18 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | Advanced Healthcare Solutions |
| Substance Name | DIBASIC POTASSIUM PHOSPHATE; SODIUM SULFATE; CHASTE TREE FRUIT; CHELIDONIUM MAJUS WHOLE; FUCUS VESICULOSUS; LYCOPODIUM CLAVATUM SPORE; THUJA OCCIDENTALIS LEAFY TWIG; CAPSICUM; MILK THISTLE; CYSTEINE; CENTELLA ASIATICA WHOLE; POTASSIUM CARBONATE; STRYCHNOS NUX-VOMICA SEED; PHYTOLACCA AMERICANA ROOT; ROSA CANINA FLOWER; SALIX ALBA BARK; GREEN TEA LEAF; GAMBOGE; ACTIVATED CHARCOAL; OYSTER SHELL CALCIUM CARBONATE, CRUDE; GRAPHITE; SODIUM CHLORIDE; SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE; SUS SCROFA ADRENAL GLAND; PORK LIVER; SUS SCROFA PANCREAS; BOS TAURUS PITUITARY GLAND, POSTERIOR; SUS SCROFA PLACENTA; THYROID, BOVINE; GYMNEMA SYLVESTRE LEAF; WATER |
| Active Ingredient Strength | 3 [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_C]/mL; [hp_C]/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 70327-0002-1
60 mL in 1 BOTTLE, DROPPER (70327-0002-1)
| Marketing Start Date | 2020-08-18 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Natural Fit" or generic name "Kali Phosphoricum, Natrum Sulphuricum, Agnus Castus, Chelidonium Majus, Fucus Vesiculosus, Lycopodium Clavatum, Thuja Occidentalis, Capsicum Annuum, Carduus Marianus, Cysteinum, Hydrocotyle Asiatica, Kali Carbonicum, Nux Vomica, Phytolacca Decandra, Rosa Canina, Flos, Salix Alba, Thea Sinensis, Gambogia, Carbo Vegetabilis, Calcarea Carbonica, Graphites, Natrum Muriaticum, Natrum Phosphoricum, Glandula Suprarenalis Suis, Hepar Suis, Pancreas Suis, Pituitarum Posterium (bovine), Placenta Totalis Suis,"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 70327-0001 | Natural Fit | Kali Phosphoricum, Natrum Sulphuricum, Agnus Castus, Chelidonium Majus, Fucus Vesiculosus, Lycopodium Clavatum, Thuja Occidentalis, Capsicum Annuum, Carduus Marianus, Cysteinum, Gambogia, Hydrocotyle Asiatica, Kali Carbonicum, Nux Vomica, Phytolacca Decandra, Rosa Canina, Flos, Salix Alba, Thea Sinensis, Carbo Vegetabilis, Calcarea Carbonica, Graphites, Natrum Muriaticum, Natrum Phosphoricum, Glandula Suprarenalis Suis, Hepar Suis, Pancreas Suis, Pituitarum Posterium (Bovine), Placenta Totalis Suis |
| 70327-0002 | Natural Fit | Kali Phosphoricum, Natrum Sulphuricum, Agnus Castus, Chelidonium Majus, Fucus Vesiculosus, Lycopodium Clavatum, Thuja Occidentalis, Capsicum Annuum, Carduus Marianus, Cysteinum, Hydrocotyle Asiatica, Kali Carbonicum, Nux Vomica, Phytolacca Decandra, Rosa Canina, Flos, Salix Alba, Thea Sinensis, Gambogia, Carbo Vegetabilis, Calcarea Carbonica, Graphites, Natrum Muriaticum, Natrum Phosphoricum, Glandula Suprarenalis Suis, Hepar Suis, Pancreas Suis, Pituitarum Posterium (Bovine), Placenta Totalis Suis, |
Trademark Results [Natural Fit]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.