NDC 72988-0031
Mentholatum
Methyl Salicylate, Dl-menthol
Mentholatum is a Topical Lotion in the Human Otc Drug category. It is labeled and distributed by Lydia Co., Ltd.. The primary component is Methyl Salicylate; Racementhol.
| Product ID | 72988-0031_e9db590f-b199-3fe8-e053-2995a90a4e74 |
| NDC | 72988-0031 |
| Product Type | Human Otc Drug |
| Proprietary Name | Mentholatum |
| Generic Name | Methyl Salicylate, Dl-menthol |
| Dosage Form | Lotion |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2022-09-30 |
| Marketing Category | UNAPPROVED DRUG OTHER / |
| Labeler Name | Lydia Co., Ltd. |
| Substance Name | METHYL SALICYLATE; RACEMENTHOL |
| Active Ingredient Strength | 0 mg/mL; mg/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 72988-0031-1
75 mL in 1 BOTTLE (72988-0031-1)
| Marketing Start Date | 2022-09-30 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Mentholatum" or generic name "Methyl Salicylate, Dl-menthol"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 10742-0002 | Mentholatum | camphor, menthol |
| 72689-0014 | MENTHOLATUM | DL-Menthol, Methyl Salicylate |
| 72988-0031 | Mentholatum | Methyl Salicylate, DL-Menthol |
Trademark Results [Mentholatum]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MENTHOLATUM 74326218 1787807 Live/Registered |
Mentholatum Company, The 1992-10-27 |
 MENTHOLATUM 73314190 1228107 Dead/Cancelled |
Mentholatum Company, The 1981-06-11 |
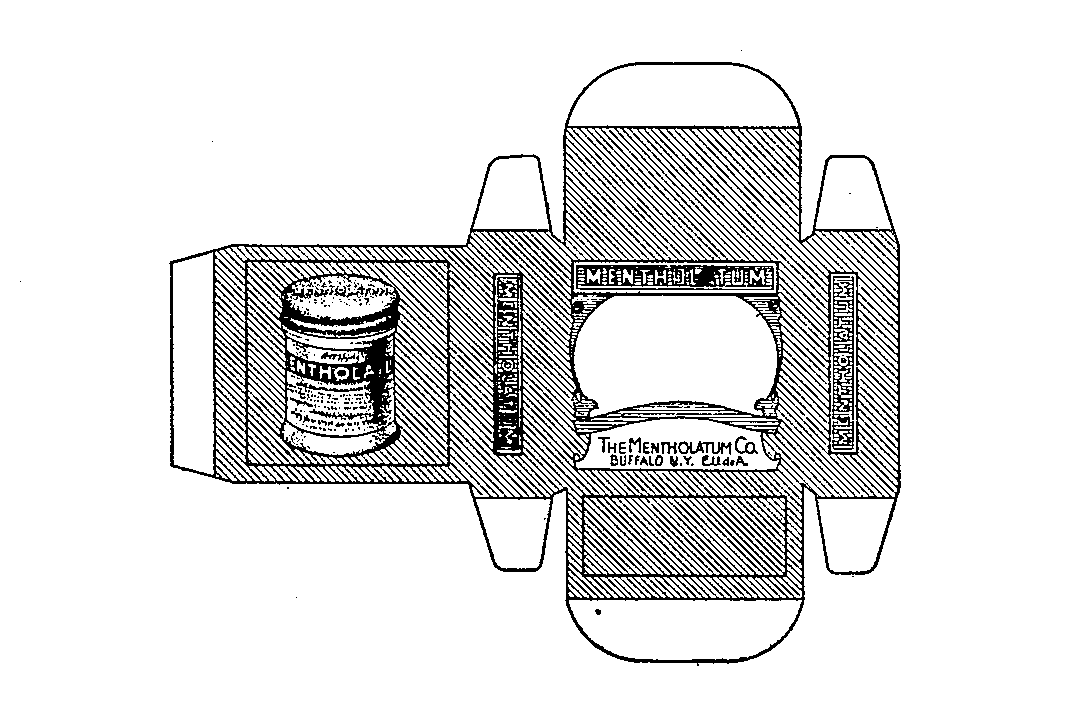 MENTHOLATUM 71266386 0248543 Dead/Expired |
1928-05-14 |
 MENTHOLATUM 71257806 0246955 Dead/Expired |
1927-11-19 |
 MENTHOLATUM 71002041 0047783 Live/Registered |
YUCCA CO., THE 1905-04-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.