NDC 73333-059
Moxe
Citrus Hand Sanitizer 4oz
Moxe is a Topical Gel in the Human Otc Drug category. It is labeled and distributed by Nutrix International, Llc.. The primary component is Alcohol.
| Product ID | 73333-059_a5402b41-f9bb-2022-e053-2a95a90a4e5d |
| NDC | 73333-059 |
| Product Type | Human Otc Drug |
| Proprietary Name | Moxe |
| Generic Name | Citrus Hand Sanitizer 4oz |
| Dosage Form | Gel |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2020-05-11 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / OTC MONOGRAPH NOT FINAL |
| Application Number | part333A |
| Labeler Name | Nutrix International, LLC. |
| Substance Name | ALCOHOL |
| Active Ingredient Strength | 1 g/59g |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 73333-059-01
59 g in 1 BOTTLE (73333-059-01)
| Marketing Start Date | 2020-05-11 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 73333-059-01 [73333005901]
Moxe GEL
| Marketing Category | OTC monograph not final |
| Application Number | part333A |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2020-05-11 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ALCOHOL | .7 g/59g |
NDC Crossover Matching brand name "Moxe" or generic name "Citrus Hand Sanitizer 4oz"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 73333-011 | Moxe | Citrus Hand Sanitizer 4oz |
| 73333-059 | Moxe | Citrus Hand Sanitizer 4oz |
| 73333-340 | Moxe | Citrus Hand Sanitizer 12oz |
Trademark Results [Moxe]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
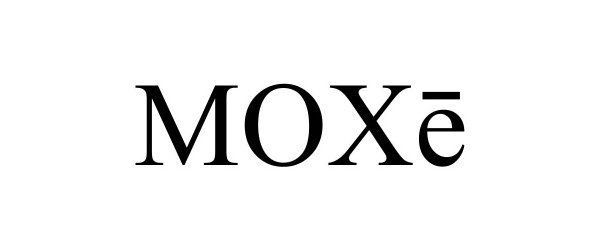 MOXE 97773509 not registered Live/Pending |
US Foods, Inc. 2023-01-30 |
 MOXE 90731126 not registered Live/Pending |
BE MOXE, LLC 2021-05-24 |
 MOXE 87979264 not registered Live/Pending |
BE MOXE, LLC 2018-02-28 |
 MOXE 87814535 5897966 Live/Registered |
BE MOXE, LLC 2018-02-28 |
 MOXE 87791988 not registered Dead/Abandoned |
NV Nutrition, LLC 2018-02-09 |
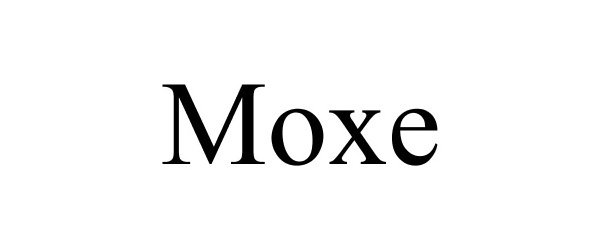 MOXE 85691154 4308837 Live/Registered |
MOXE HEALTH CORPORATION 2012-07-31 |
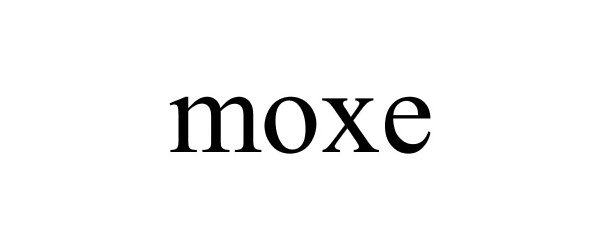 MOXE 77940172 not registered Dead/Abandoned |
Moxe, LLC 2010-02-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.