NDC 76472-3002
APIS MELLIFERA 200ck
Apis Mellifera
APIS MELLIFERA 200ck is a Sublingual Pellet in the Human Otc Drug category. It is labeled and distributed by Sevene Usa. The primary component is Apis Mellifera.
| Product ID | 76472-3002_c8c3e1c1-a801-6a01-e053-2995a90a8056 |
| NDC | 76472-3002 |
| Product Type | Human Otc Drug |
| Proprietary Name | APIS MELLIFERA 200ck |
| Generic Name | Apis Mellifera |
| Dosage Form | Pellet |
| Route of Administration | SUBLINGUAL |
| Marketing Start Date | 2011-11-23 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / |
| Labeler Name | SEVENE USA |
| Substance Name | APIS MELLIFERA |
| Active Ingredient Strength | 200 [kp_C]/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 76472-3002-1
80 PELLET in 1 CYLINDER (76472-3002-1)
| Marketing Start Date | 2011-11-23 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "APIS MELLIFERA 200ck" or generic name "Apis Mellifera"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 76472-1125 | APIS MELLIFERA | APIS MELLIFERA |
| 76472-3002 | APIS MELLIFERA | APIS MELLIFERA |
| 76472-4002 | APIS MELLIFERA | APIS MELLIFERA |
| 0220-0399 | Apis mellifica | APIS MELLIFERA |
| 0220-0400 | Apis mellifica | APIS MELLIFERA |
| 0220-0403 | Apis mellifica | APIS MELLIFERA |
| 0220-0404 | Apis mellifica | APIS MELLIFERA |
| 0220-0406 | Apis mellifica | APIS MELLIFERA |
| 0220-0408 | Apis mellifica | APIS MELLIFERA |
| 0220-0409 | Apis mellifica | APIS MELLIFERA |
| 0220-0410 | Apis mellifica | APIS MELLIFERA |
| 0220-0411 | Apis mellifica | APIS MELLIFERA |
| 0220-0412 | Apis mellifica | APIS MELLIFERA |
| 0220-0413 | Apis mellifica | APIS MELLIFERA |
| 0220-0417 | Apis mellifica | APIS MELLIFERA |
| 54973-0603 | APIS MELLIFICA | apis mellifera |
| 54973-2903 | APIS MELLIFICA | apis mellifera |
| 68428-214 | Apis mellifica | APIS MELLIFERA |
| 71919-066 | Apis mellifica | APIS MELLIFERA |
| 68428-030 | Apis Mellifica Kit Refill | APIS MELLIFERA |
| 68428-080 | Apis Mellifica Kit Refill | APIS MELLIFERA |
| 64117-953 | Stings, Bites, Swellings | APIS MELLIFERA |
Trademark Results [APIS MELLIFERA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
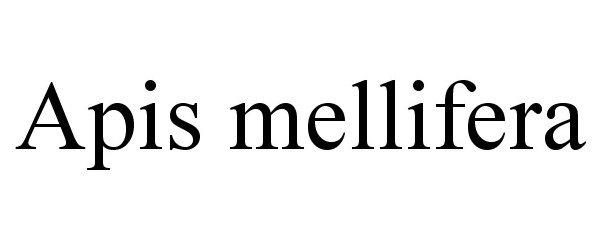 APIS MELLIFERA 87264792 5241247 Live/Registered |
Nanjing Apis Mellifera Co. Ltd 2016-12-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.