NDC 76484-948
20/20 Topical Anesthetic
Benzocaine
20/20 Topical Anesthetic is a Dental Strip in the Human Otc Drug category. It is labeled and distributed by Advanced Dental Anesthetics. The primary component is Benzocaine.
| Product ID | 76484-948_55480e63-4b5c-4230-905b-5edfc1f7bb18 |
| NDC | 76484-948 |
| Product Type | Human Otc Drug |
| Proprietary Name | 20/20 Topical Anesthetic |
| Generic Name | Benzocaine |
| Dosage Form | Strip |
| Route of Administration | DENTAL |
| Marketing Start Date | 2012-02-28 |
| Marketing Category | OTC MONOGRAPH FINAL / OTC MONOGRAPH FINAL |
| Application Number | part356 |
| Labeler Name | Advanced Dental Anesthetics |
| Substance Name | BENZOCAINE |
| Active Ingredient Strength | 10 mg/1 |
| NDC Exclude Flag | E |
| Listing Certified Through | 2017-12-31 |
Packaging
NDC 76484-948-24
1 CASE in 1 POUCH (76484-948-24) > 24 STRIP in 1 CASE
| Marketing Start Date | 2012-02-28 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 76484-948-24 [76484094824]
20/20 Topical Anesthetic STRIP
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part356 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2012-02-28 |
| Inactivation Date | 2019-11-13 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| BENZOCAINE | 10 mg/1 |
OpenFDA Data
| SPL SET ID: | 0584e235-34f8-4d53-aa7c-ac11e01ac456 |
| Manufacturer | |
| UNII | |
| UPC Code |
NDC Crossover Matching brand name "20/20 Topical Anesthetic" or generic name "Benzocaine"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 76484-948 | 20/20 Topical Anesthetic | 20/20 Topical Anesthetic |
| 0283-0220 | HurriCaine | Benzocaine |
| 0283-0293 | HurriCaine | Benzocaine |
| 0283-0520 | HurriCaine | Benzocaine |
| 0283-0569 | HurriCaine | Benzocaine |
| 0283-0679 | HurriCaine | Benzocaine |
| 0283-0871 | HurriCaine | BENZOCAINE |
| 0283-0886 | HurriCaine | Benzocaine |
| 0283-0914 | HurriCaine | Benzocaine |
| 0283-0920 | HurriCaine | Benzocaine |
| 0283-0998 | HurriCaine | Benzocaine |
| 0283-1016 | HurriCaine | BENZOCAINE |
Trademark Results [20/20]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
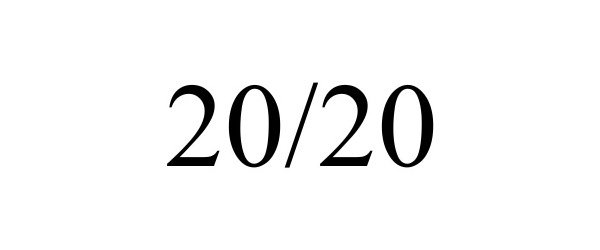 20/20 98157766 not registered Live/Pending |
Veriphy Inc 2023-08-30 |
 20/20 90196808 not registered Live/Pending |
20/20 Company 2020-09-21 |
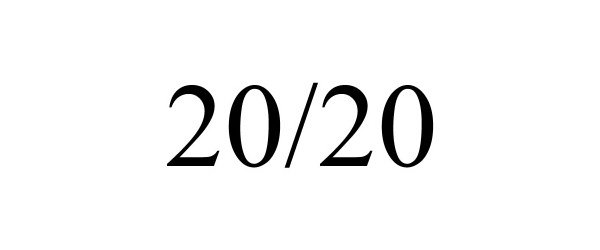 20/20 90148497 not registered Live/Pending |
RT RECOVER INNOVATIONS LTD 2020-08-31 |
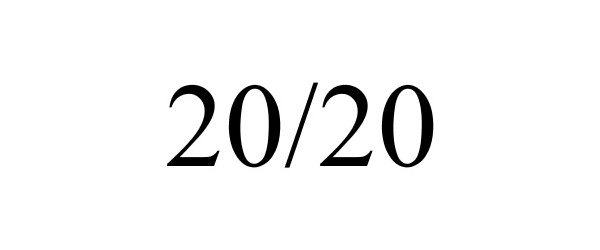 20/20 88380297 not registered Live/Pending |
20/20 Company 2019-04-10 |
 20/20 88107448 not registered Live/Pending |
Thor-lo, Inc. 2018-09-06 |
 20/20 87755296 not registered Dead/Abandoned |
20/20 IP LLC 2018-01-15 |
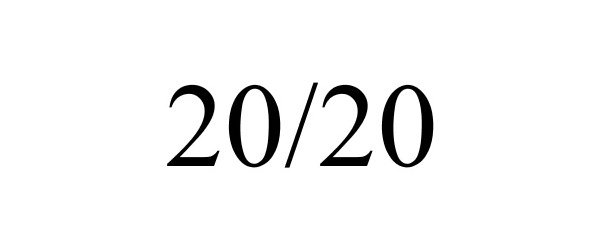 20/20 87655457 not registered Live/Pending |
Jobson Medical Information LLC 2017-10-23 |
 20/20 87522139 5406224 Live/Registered |
Jobson Medical Information LLC 2017-07-10 |
 20/20 86790405 not registered Dead/Abandoned |
Performance Support Systems, Inc. 2015-10-16 |
 20/20 86055696 4736017 Live/Registered |
ASHLAND LICENSING & INTELLECTUAL PROPERTY LLC 2013-09-04 |
 20/20 85892291 not registered Dead/Abandoned |
PRO GLASS ALLIANCE, LLC 2013-04-01 |
 20/20 85543262 4300285 Live/Registered |
Summit Electric Supply Company, Inc. 2012-02-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.