NDC 78670-130
Symjepi
Epinephrine
Symjepi is a Intramuscular; Subcutaneous Injection, Solution in the Human Prescription Drug category. It is labeled and distributed by Uswm, Llc. The primary component is Epinephrine.
| Product ID | 78670-130_35d5acb0-20da-47b9-abb5-d645b7b5048c |
| NDC | 78670-130 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Symjepi |
| Generic Name | Epinephrine |
| Dosage Form | Injection, Solution |
| Route of Administration | INTRAMUSCULAR; SUBCUTANEOUS |
| Marketing Start Date | 2020-11-01 |
| Marketing Category | NDA / NDA |
| Application Number | NDA207534 |
| Labeler Name | USWM, LLC |
| Substance Name | EPINEPHRINE |
| Active Ingredient Strength | 0 mg/.3mL |
| Pharm Classes | Adrenergic alpha-Agonists [MoA],Adrenergic beta-Agonists [MoA],alpha-Adrenergic Agonist [EPC],beta-Adrenergic Agonist [EPC],Catecholamine [EPC],Catecholamines [CS] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 78670-130-02
2 SYRINGE in 1 CARTON (78670-130-02) > .3 mL in 1 SYRINGE (78670-130-11)
| Marketing Start Date | 2020-11-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Symjepi" or generic name "Epinephrine"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0781-3442 | SYMJEPI | Epinephrine |
| 0781-3448 | SYMJEPI | Epinephrine |
| 38739-200 | SYMJEPI | SYMJEPI |
| 78670-131 | Symjepi | Symjepi |
| 78670-130 | Symjepi | Symjepi |
| 0220-0076 | Adrenalinum | EPINEPHRINE |
| 0220-0078 | Adrenalinum | EPINEPHRINE |
| 0093-5985 | Epinephrine | Epinephrine |
| 0093-5986 | Epinephrine | Epinephrine |
| 0115-1694 | epinephrine | epinephrine |
| 0115-1695 | epinephrine | epinephrine |
| 0404-9856 | Epinephrine | Epinephrine |
| 0404-9857 | EPINEPHRINE | EPINEPHRINE |
| 0409-4921 | Epinephrine | EPINEPHRINE |
| 0409-4933 | EPINEPHRINE | epinephrine |
| 0409-7241 | EPINEPHRINE | epinephrine |
| 0517-1130 | Epinephrine | Epinephrine |
| 0517-1171 | epinephrine | epinephrine |
Trademark Results [Symjepi]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
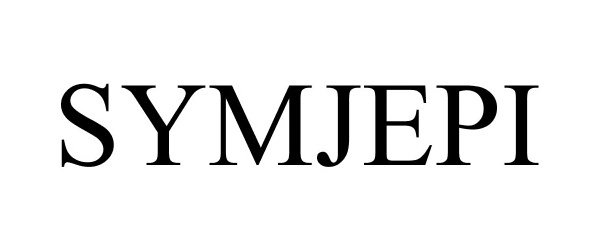 SYMJEPI 88162331 5853281 Live/Registered |
Adamis Pharmaceuticals Corporation 2018-10-19 |
 SYMJEPI 86582435 not registered Dead/Abandoned |
Adamis Pharmaceuticals Corporation 2015-03-31 |
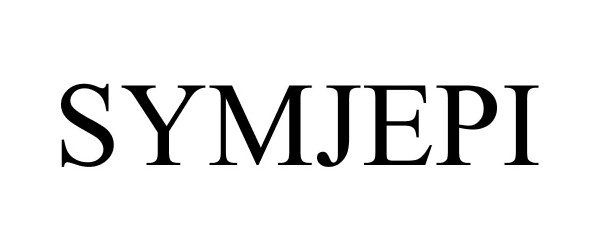 SYMJEPI 86582421 not registered Dead/Abandoned |
Adamis Pharmaceuticals Corporation 2015-03-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.