NDC 82718-1110
Blockaid
Lidocaine, Tetracaine
Blockaid is a Topical Cream in the Human Otc Drug category. It is labeled and distributed by Softap Inc. The primary component is Lidocaine; Tetracaine.
| Product ID | 82718-1110_de4b93cc-1f65-1a4a-e053-2995a90a48a5 |
| NDC | 82718-1110 |
| Product Type | Human Otc Drug |
| Proprietary Name | Blockaid |
| Generic Name | Lidocaine, Tetracaine |
| Dosage Form | Cream |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2022-05-06 |
| Marketing Category | UNAPPROVED DRUG OTHER / |
| Labeler Name | SofTap Inc |
| Substance Name | LIDOCAINE; TETRACAINE |
| Active Ingredient Strength | 0 g/10g; g/10g |
| Pharm Classes | Amide Local Anesthetic [EPC], Amides [CS], Antiarrhythmic [EPC], Ester Local Anesthetic [EPC], Esters [CS], Local Anesthesia [PE], Local Anesthesia [PE] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 82718-1110-1
10 g in 1 TUBE (82718-1110-1)
| Marketing Start Date | 2022-05-06 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Blockaid" or generic name "Lidocaine, Tetracaine"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 82718-1110 | Blockaid | Lidocaine, Tetracaine |
| 82171-001 | Ritual Tattoo | Lidocaine, Tetracaine |
Trademark Results [Blockaid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
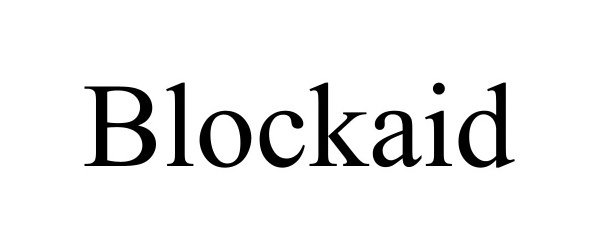 BLOCKAID 98120245 not registered Live/Pending |
Blockaid Ltd. 2023-08-07 |
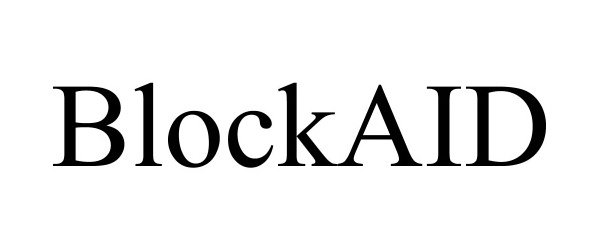 BLOCKAID 88900141 not registered Live/Pending |
Zenwise LLC 2020-05-04 |
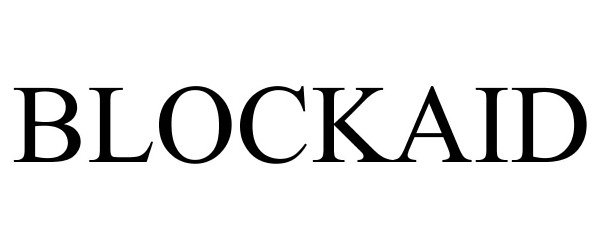 BLOCKAID 85498545 4973352 Live/Registered |
Blockaid Pty Ltd 2011-12-19 |
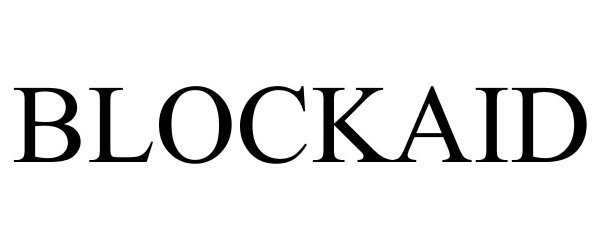 BLOCKAID 78798998 not registered Dead/Abandoned |
Great Southern Wood Preserving, Inc. 2006-01-25 |
 BLOCKAID 78699743 3353596 Live/Registered |
Acoustics First Corporation 2005-08-24 |
 BLOCKAID 78432237 3034674 Live/Registered |
Alexis A. Lawson and Michael R. LawsonPartnership, The 2004-06-09 |
 BLOCKAID 76581996 not registered Dead/Abandoned |
Great Southern Wood Preserving, Inc. 2004-03-17 |
 BLOCKAID 75936284 2592436 Dead/Cancelled |
INVERNESS MEDICAL - BIOSTAR INC. INVERNESS MEDICAL PROFESSIONAL DIAGNOSTICS 2000-03-06 |
 BLOCKAID 74556501 1908310 Live/Registered |
Biomet, Inc. 1994-08-02 |
 BLOCKAID 73664526 1472503 Dead/Cancelled |
RIDDELL, INC. 1987-06-04 |
 BLOCKAID 72231113 0827088 Dead/Expired |
GLIDDEN COMPANY, THE 1965-10-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.