SAMPLOK
Tubes, Vials, Systems, Serum Separators, Blood Collection
ITL CORPORATION, PTY LTD.
The following data is part of a premarket notification filed by Itl Corporation, Pty Ltd. with the FDA for Samplok.
Pre-market Notification Details
| Device ID | K000777 |
| 510k Number | K000777 |
| Device Name: | SAMPLOK |
| Classification | Tubes, Vials, Systems, Serum Separators, Blood Collection |
| Applicant | ITL CORPORATION, PTY LTD. 15825 SHADY GROVE RD. SUITE 90 Rockville, MD 20850 |
| Contact | Kenneth A Palmer |
| Correspondent | Kenneth A Palmer ITL CORPORATION, PTY LTD. 15825 SHADY GROVE RD. SUITE 90 Rockville, MD 20850 |
| Product Code | JKA |
| CFR Regulation Number | 862.1675 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2000-03-09 |
| Decision Date | 2000-04-11 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 19555240401226 | K000777 | 000 |
| 19555240401523 | K000777 | 000 |
| 19555240401530 | K000777 | 000 |
| 19555240401547 | K000777 | 000 |
| 19555240401554 | K000777 | 000 |
| 19555240400021 | K000777 | 000 |
| 19555240400069 | K000777 | 000 |
| 19555240400175 | K000777 | 000 |
| 19555240400205 | K000777 | 000 |
| 19555240401301 | K000777 | 000 |
Trademark Results [SAMPLOK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
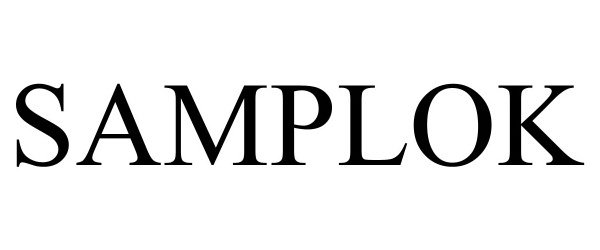 SAMPLOK 98099921 not registered Live/Pending |
Noble House Group Pty. Ltd. 2023-07-25 |
 SAMPLOK 75881897 2756567 Live/Registered |
Noble House Group Pty Ltd. 1999-12-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.