INSIDE VIEW
Endoscope, Rigid
MONADNOCK OPTICS, INC.
The following data is part of a premarket notification filed by Monadnock Optics, Inc. with the FDA for Inside View.
Pre-market Notification Details
| Device ID | K001217 |
| 510k Number | K001217 |
| Device Name: | INSIDE VIEW |
| Classification | Endoscope, Rigid |
| Applicant | MONADNOCK OPTICS, INC. 1464 HOLCOMB RD. Huntingdon Valley, PA 19006 |
| Contact | Leonard J Bonnell |
| Correspondent | Leonard J Bonnell MONADNOCK OPTICS, INC. 1464 HOLCOMB RD. Huntingdon Valley, PA 19006 |
| Product Code | GCM |
| CFR Regulation Number | 876.1500 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2000-04-17 |
| Decision Date | 2000-07-11 |
Trademark Results [INSIDE VIEW]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INSIDE VIEW 85916312 not registered Dead/Abandoned |
Citigroup Inc. 2013-04-26 |
 INSIDE VIEW 80996776 0996776 Dead/Cancelled |
Harriet Schoenthal, Inc. 0000-00-00 |
 INSIDE VIEW 77813795 not registered Dead/Abandoned |
Citigroup Inc. 2009-08-27 |
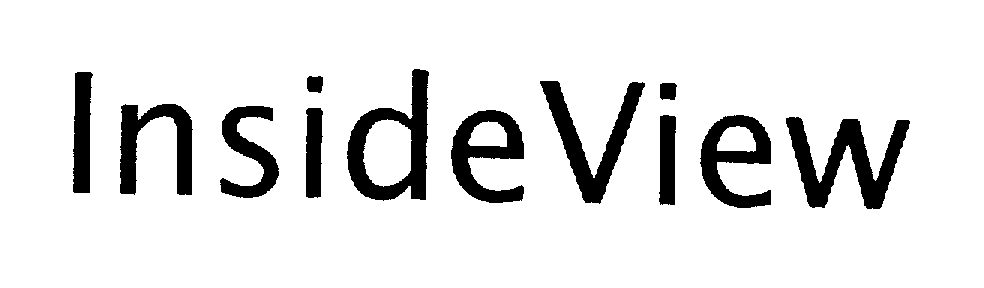 INSIDE VIEW 75485868 2276892 Dead/Cancelled |
Geometric Technology Systems, Inc. 1998-05-15 |
 INSIDE VIEW 74563475 1913304 Dead/Cancelled |
Republic Pictures Corporation 1994-08-19 |
 INSIDE VIEW 74457700 1870138 Dead/Cancelled |
INSIDE VIEW INCORPORATED 1993-11-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.