LINKMAX
Cement, Dental
GC AMERICA, INC.
The following data is part of a premarket notification filed by Gc America, Inc. with the FDA for Linkmax.
Pre-market Notification Details
| Device ID | K001823 |
| 510k Number | K001823 |
| Device Name: | LINKMAX |
| Classification | Cement, Dental |
| Applicant | GC AMERICA, INC. 3737 WEST 127TH ST. Alsip, IL 60803 |
| Contact | Terry L Joritz |
| Correspondent | Terry L Joritz GC AMERICA, INC. 3737 WEST 127TH ST. Alsip, IL 60803 |
| Product Code | EMA |
| CFR Regulation Number | 872.3275 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2000-06-16 |
| Decision Date | 2000-08-16 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 04548161297304 | K001823 | 000 |
| 14548161282611 | K001823 | 000 |
Trademark Results [LINKMAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
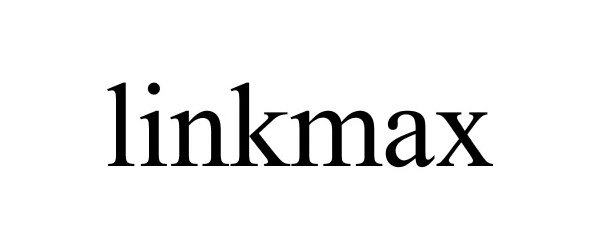 LINKMAX 87848676 not registered Dead/Abandoned |
Ningbo Nuofo E-commerce Co.,Ltd. 2018-03-25 |
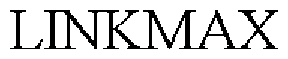 LINKMAX 79056362 3663815 Dead/Cancelled |
CASTROL LIMITED 2008-01-17 |
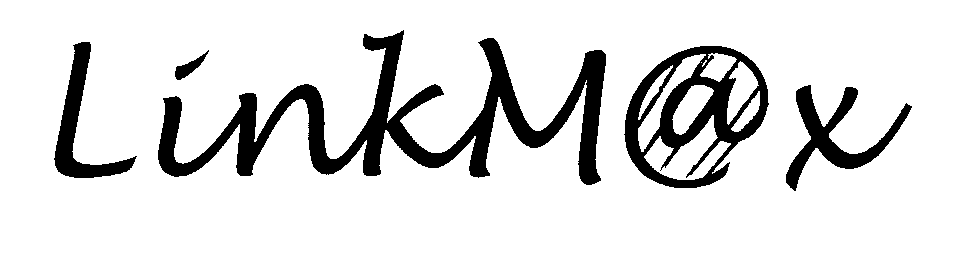 LINKMAX 76392002 2777399 Dead/Cancelled |
BroadMax Technologies, Inc. 2002-04-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.