OXYGENATOR
Chamber, Oxygen, Topical, Extremity
ADJUNCTIVE THERAPEUTICS, INC.
The following data is part of a premarket notification filed by Adjunctive Therapeutics, Inc. with the FDA for Oxygenator.
Pre-market Notification Details
| Device ID | K002632 |
| 510k Number | K002632 |
| Device Name: | OXYGENATOR |
| Classification | Chamber, Oxygen, Topical, Extremity |
| Applicant | ADJUNCTIVE THERAPEUTICS, INC. 10 SCOTCH MIST COURT Potomac, MD 20854 |
| Contact | Myron Z Bernstein |
| Correspondent | Myron Z Bernstein ADJUNCTIVE THERAPEUTICS, INC. 10 SCOTCH MIST COURT Potomac, MD 20854 |
| Product Code | KPJ |
| CFR Regulation Number | 878.5650 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2000-08-23 |
| Decision Date | 2001-10-25 |
Trademark Results [OXYGENATOR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
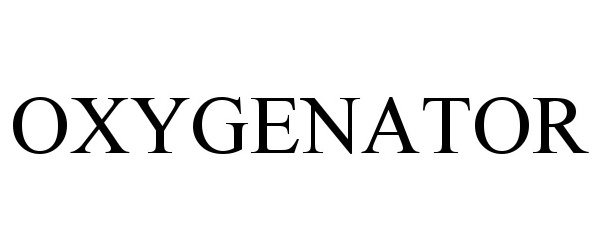 OXYGENATOR 97797936 not registered Live/Pending |
T-H Marine Supplies, LLC 2023-02-16 |
 OXYGENATOR 87599987 5388706 Live/Registered |
T-H Marine Supplies, Inc. 2017-09-07 |
 OXYGENATOR 78974798 not registered Dead/Abandoned |
International Business Development, Inc. 2006-09-14 |
 OXYGENATOR 78381314 not registered Dead/Abandoned |
Global Healing Center, Inc. 2004-03-09 |
 OXYGENATOR 76223773 not registered Dead/Abandoned |
Plank, David 2001-03-14 |
 OXYGENATOR 75347782 not registered Dead/Abandoned |
H2O Technologies, Ltd. 1997-08-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.