TAKE 1
Material, Impression
SYBRON DENTAL SPECIALTIES, INC.
The following data is part of a premarket notification filed by Sybron Dental Specialties, Inc. with the FDA for Take 1.
Pre-market Notification Details
| Device ID | K003808 |
| 510k Number | K003808 |
| Device Name: | TAKE 1 |
| Classification | Material, Impression |
| Applicant | SYBRON DENTAL SPECIALTIES, INC. 1717 WEST COLLINS AVE. Orange, CA 92867 |
| Contact | Colleen Boswell |
| Correspondent | Colleen Boswell SYBRON DENTAL SPECIALTIES, INC. 1717 WEST COLLINS AVE. Orange, CA 92867 |
| Product Code | ELW |
| CFR Regulation Number | 872.3660 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2000-12-08 |
| Decision Date | 2001-03-01 |
Trademark Results [TAKE 1]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
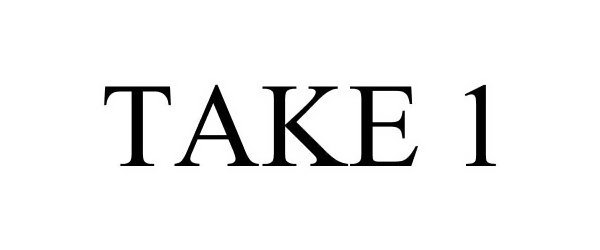 TAKE 1 85107222 4068240 Live/Registered |
Elite Brands Inc. 2010-08-13 |
 TAKE 1 78883883 not registered Dead/Abandoned |
Take 1 Technologies, LLC 2006-05-15 |
 TAKE 1 75525778 2352682 Dead/Cancelled |
Kerr Corporation 1998-07-27 |
 TAKE 1 75165667 2203488 Dead/Cancelled |
AUDIO PRODUCTS INTERNATIONAL CORP. 1996-09-12 |
 TAKE 1 73772328 1651357 Dead/Cancelled |
TONG YANG POLYESTER CO., LTD. 1988-12-30 |
 TAKE 1 73311986 1209819 Dead/Cancelled |
Take 1 Sportswear, Inc. 1981-05-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.