IBD-CHEK
Lactoferrin, Antigen, Antiserum, Control
TECHLAB, INC.
The following data is part of a premarket notification filed by Techlab, Inc. with the FDA for Ibd-chek.
Pre-market Notification Details
| Device ID | K011396 |
| 510k Number | K011396 |
| Device Name: | IBD-CHEK |
| Classification | Lactoferrin, Antigen, Antiserum, Control |
| Applicant | TECHLAB, INC. 1861 PRATT DR., STE. 1030 Blacksburg, VA 24060 -6364 |
| Contact | David M Lyerly |
| Correspondent | David M Lyerly TECHLAB, INC. 1861 PRATT DR., STE. 1030 Blacksburg, VA 24060 -6364 |
| Product Code | DEG |
| CFR Regulation Number | 866.5570 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2001-05-07 |
| Decision Date | 2001-06-18 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00857031002271 | K011396 | 000 |
| 00857031002080 | K011396 | 000 |
Trademark Results [IBD-CHEK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
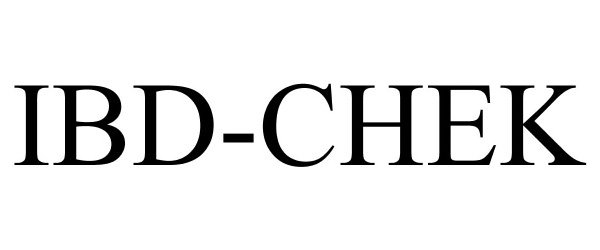 IBD-CHEK 77333068 3504161 Dead/Cancelled |
TechLab, Inc. 2007-11-19 |
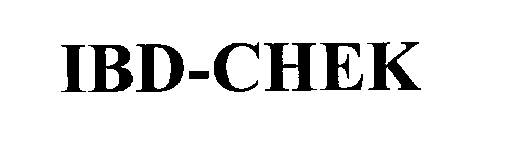 IBD-CHEK 76276405 2664800 Live/Registered |
TechLab, Inc. 2001-06-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.