SURE-LOK
Needle, Hypodermic, Single Lumen
VITAL SIGNS, INC.
The following data is part of a premarket notification filed by Vital Signs, Inc. with the FDA for Sure-lok.
Pre-market Notification Details
| Device ID | K021315 |
| 510k Number | K021315 |
| Device Name: | SURE-LOK |
| Classification | Needle, Hypodermic, Single Lumen |
| Applicant | VITAL SIGNS, INC. 11039 EAST LANSING CIRCLE Englewood, CO 80112 |
| Contact | Thomas W Dielmann |
| Correspondent | Thomas W Dielmann VITAL SIGNS, INC. 11039 EAST LANSING CIRCLE Englewood, CO 80112 |
| Product Code | FMI |
| CFR Regulation Number | 880.5570 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Abbreviated |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2002-04-25 |
| Decision Date | 2002-08-21 |
| Summary: | summary |
Trademark Results [SURE-LOK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SURE-LOK 87042656 5309128 Live/Registered |
Mollewood Export, Inc. 2016-05-19 |
 SURE-LOK 86866323 5307277 Live/Registered |
Amsino International, Inc. 2016-01-05 |
 SURE-LOK 86537165 not registered Dead/Abandoned |
Harbor Freight Tools USA, Inc. 2015-02-17 |
 SURE-LOK 86001741 not registered Dead/Abandoned |
Shop Vac Corporation 2013-07-03 |
 SURE-LOK 85366244 not registered Dead/Abandoned |
The Great American Tool Company, Inc. 2011-07-08 |
 SURE-LOK 85023902 not registered Dead/Abandoned |
Trinity Logistics Corporation 2010-04-27 |
 SURE-LOK 78405039 2966899 Live/Registered |
SALISBURY ELECTRICAL SAFETY L.L.C. 2004-04-20 |
 SURE-LOK 77290180 not registered Dead/Abandoned |
The Great American Tool Company, Inc. 2007-09-27 |
 SURE-LOK 76611674 not registered Dead/Abandoned |
Woodhead Industries, Inc. 2004-09-16 |
 SURE-LOK 76376791 3061464 Dead/Cancelled |
PAK-RITE, LTD.-MICHIGAN 2002-02-28 |
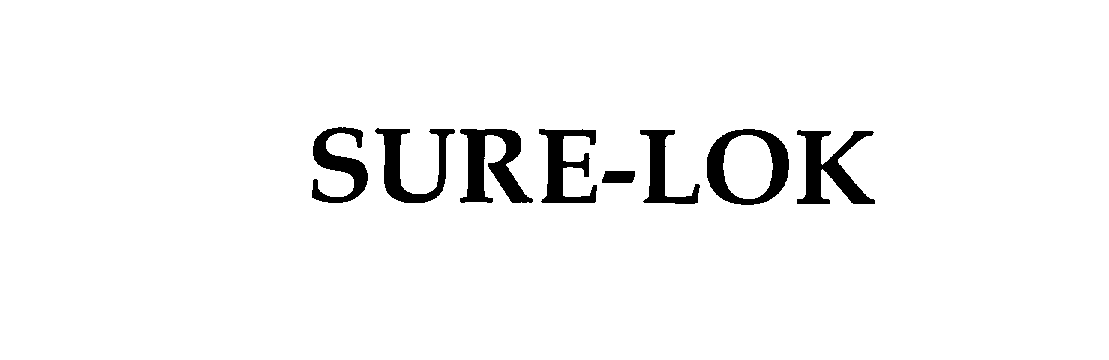 SURE-LOK 76069431 not registered Dead/Abandoned |
VITAL SIGNS, INC. 2000-06-12 |
 SURE-LOK 75911236 2793447 Live/Registered |
SURE-LOK INTERNATIONAL, LLC 2000-02-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.