BI-OSTETIC
Filler, Bone Void, Calcium Compound
BERKELEY ADVANCED BIOMATERIALS, INC.
The following data is part of a premarket notification filed by Berkeley Advanced Biomaterials, Inc. with the FDA for Bi-ostetic.
Pre-market Notification Details
| Device ID | K023703 |
| 510k Number | K023703 |
| Device Name: | BI-OSTETIC |
| Classification | Filler, Bone Void, Calcium Compound |
| Applicant | BERKELEY ADVANCED BIOMATERIALS, INC. 1933 DAVIS ST. SUITE 307 San Leandro, CA 94577 |
| Contact | Francois Genin |
| Correspondent | Francois Genin BERKELEY ADVANCED BIOMATERIALS, INC. 1933 DAVIS ST. SUITE 307 San Leandro, CA 94577 |
| Product Code | MQV |
| CFR Regulation Number | 888.3045 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2002-11-04 |
| Decision Date | 2003-01-30 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00816125021628 | K023703 | 000 |
| 00858418003300 | K023703 | 000 |
| 00858418003294 | K023703 | 000 |
| 00858418003256 | K023703 | 000 |
| 00858418003249 | K023703 | 000 |
| 00858418003232 | K023703 | 000 |
| 00858418003010 | K023703 | 000 |
| 00858418003003 | K023703 | 000 |
| 00816125024766 | K023703 | 000 |
| 00816125025640 | K023703 | 000 |
| 00816125025633 | K023703 | 000 |
| 00816125025626 | K023703 | 000 |
| 00816125025619 | K023703 | 000 |
| 00816125025602 | K023703 | 000 |
| 00816125025596 | K023703 | 000 |
| 00816125025589 | K023703 | 000 |
| 00816125025572 | K023703 | 000 |
| 00858418003324 | K023703 | 000 |
| 00858418003331 | K023703 | 000 |
| 00858418003348 | K023703 | 000 |
| 00816125021611 | K023703 | 000 |
| 00816125021604 | K023703 | 000 |
| 00816125021598 | K023703 | 000 |
| 00816125021581 | K023703 | 000 |
| 00816125021574 | K023703 | 000 |
| 00816125021567 | K023703 | 000 |
| 00816125021550 | K023703 | 000 |
| 00858418003966 | K023703 | 000 |
| 00858418003812 | K023703 | 000 |
| 00858418003805 | K023703 | 000 |
| 00858418003799 | K023703 | 000 |
| 00858418003782 | K023703 | 000 |
| 00858418003393 | K023703 | 000 |
| 00858418003386 | K023703 | 000 |
| 00858418003379 | K023703 | 000 |
| 00858418003362 | K023703 | 000 |
| 00816125025565 | K023703 | 000 |
Trademark Results [BI-OSTETIC]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
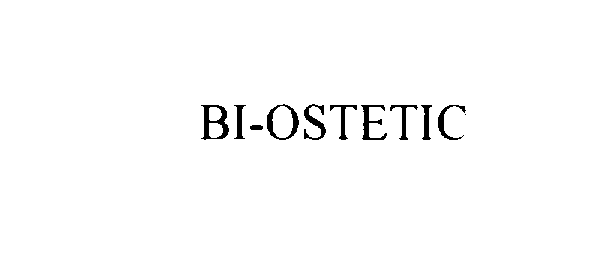 BI-OSTETIC 76097625 not registered Dead/Abandoned |
Berkeley Advanced Biomaterials, Inc. 2000-07-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.