WAGNER CONE PROSTHESIS
Prosthesis, Hip, Semi-constrained, Metal/ceramic/polymer, Cemented Or Non-porous, Uncemented
CENTERPULSE ORTHOPEDICS, INC.
The following data is part of a premarket notification filed by Centerpulse Orthopedics, Inc. with the FDA for Wagner Cone Prosthesis.
Pre-market Notification Details
| Device ID | K032380 |
| 510k Number | K032380 |
| Device Name: | WAGNER CONE PROSTHESIS |
| Classification | Prosthesis, Hip, Semi-constrained, Metal/ceramic/polymer, Cemented Or Non-porous, Uncemented |
| Applicant | CENTERPULSE ORTHOPEDICS, INC. 9900 SPECTRUM DR. Austin, TX 78717 |
| Contact | Robert M Wolfarth |
| Correspondent | Robert M Wolfarth CENTERPULSE ORTHOPEDICS, INC. 9900 SPECTRUM DR. Austin, TX 78717 |
| Product Code | LZO |
| CFR Regulation Number | 888.3353 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2003-08-01 |
| Decision Date | 2003-09-22 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00889024282308 | K032380 | 000 |
| 00889024282179 | K032380 | 000 |
| 00889024282162 | K032380 | 000 |
| 00889024282155 | K032380 | 000 |
| 00889024282148 | K032380 | 000 |
| 00889024282131 | K032380 | 000 |
| 00889024282124 | K032380 | 000 |
| 00889024282117 | K032380 | 000 |
| 00889024282100 | K032380 | 000 |
| 00889024282094 | K032380 | 000 |
| 00889024282087 | K032380 | 000 |
| 00889024282186 | K032380 | 000 |
| 00889024282193 | K032380 | 000 |
| 00889024282292 | K032380 | 000 |
| 00889024282285 | K032380 | 000 |
| 00889024282278 | K032380 | 000 |
| 00889024282261 | K032380 | 000 |
| 00889024282254 | K032380 | 000 |
| 00889024282247 | K032380 | 000 |
| 00889024282230 | K032380 | 000 |
| 00889024282223 | K032380 | 000 |
| 00889024282216 | K032380 | 000 |
| 00889024282209 | K032380 | 000 |
| 00889024282070 | K032380 | 000 |
Trademark Results [WAGNER CONE PROSTHESIS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
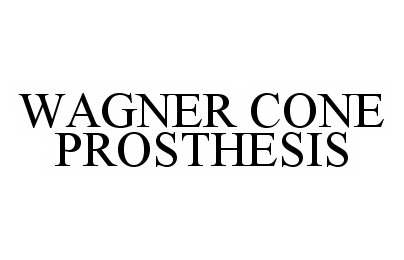 WAGNER CONE PROSTHESIS 78555762 3216384 Live/Registered |
Zimmer GmbH 2005-01-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.