VARNISHAMERICA
Varnish, Cavity
MEDICAL PRODUCTS LABORATORIES, INC.
The following data is part of a premarket notification filed by Medical Products Laboratories, Inc. with the FDA for Varnishamerica.
Pre-market Notification Details
| Device ID | K040098 |
| 510k Number | K040098 |
| Device Name: | VARNISHAMERICA |
| Classification | Varnish, Cavity |
| Applicant | MEDICAL PRODUCTS LABORATORIES, INC. 9990 GLOBAL RD. Philadelphia, PA 19115 |
| Contact | Robert J Malerman |
| Correspondent | Robert Mosenkis CITECH 5200 BUTLER PIKE Plymouth Meeting, PA 19462 -1298 |
| Product Code | LBH |
| CFR Regulation Number | 872.3260 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | Yes |
| Combination Product | No |
| Date Received | 2004-01-20 |
| Decision Date | 2004-02-04 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| D686200306770 | K040098 | 000 |
| 10889950141745 | K040098 | 000 |
| 50732224005558 | K040098 | 000 |
| 50732224005565 | K040098 | 000 |
| 50732224005572 | K040098 | 000 |
| D686200327620 | K040098 | 000 |
| D686200327630 | K040098 | 000 |
| D686200327640 | K040098 | 000 |
| D686200327650 | K040098 | 000 |
| D686200327700 | K040098 | 000 |
| D686200306730 | K040098 | 000 |
| D686200306740 | K040098 | 000 |
| D686200306750 | K040098 | 000 |
| D686200306760 | K040098 | 000 |
| 10889950141738 | K040098 | 000 |
Trademark Results [VARNISHAMERICA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
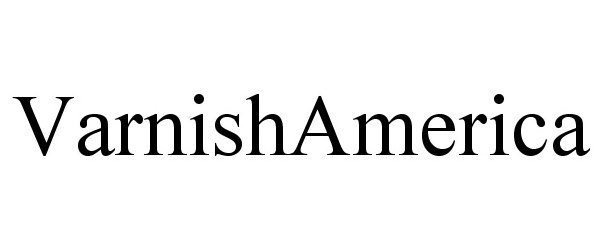 VARNISHAMERICA 86045104 4536542 Live/Registered |
Medical Products Laboratories, Inc. 2013-08-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.