SINON
Powered Laser Surgical Instrument
WAVELIGHT LASER TECHNOLOGIE AG
The following data is part of a premarket notification filed by Wavelight Laser Technologie Ag with the FDA for Sinon.
Pre-market Notification Details
| Device ID | K040433 |
| 510k Number | K040433 |
| Device Name: | SINON |
| Classification | Powered Laser Surgical Instrument |
| Applicant | WAVELIGHT LASER TECHNOLOGIE AG 5 TIMBER LANE North Reading, MA 01864 |
| Contact | Maureen O'connell |
| Correspondent | Maureen O'connell WAVELIGHT LASER TECHNOLOGIE AG 5 TIMBER LANE North Reading, MA 01864 |
| Product Code | GEX |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2004-02-19 |
| Decision Date | 2004-05-19 |
| Summary: | summary |
Trademark Results [SINON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SINON 79007659 3181080 Dead/Cancelled |
Alma Lasers Ltd. 2004-10-05 |
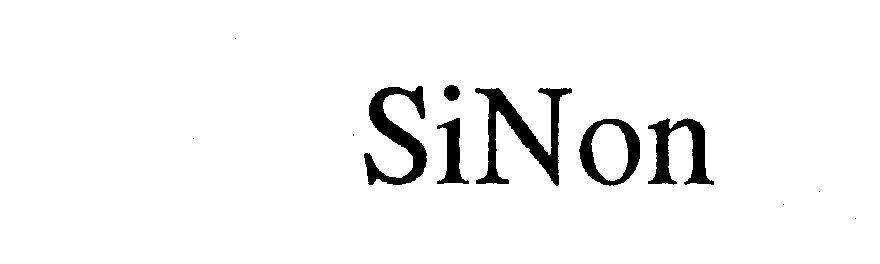 SINON 74363701 1991494 Dead/Cancelled |
Liu, Louise Yan 1993-02-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.