UNDECIDED
System, Image Processing, Radiological
SIEMENS MEDICAL SOLUTIONS USA, INC.
The following data is part of a premarket notification filed by Siemens Medical Solutions Usa, Inc. with the FDA for Undecided.
Pre-market Notification Details
| Device ID | K041484 |
| 510k Number | K041484 |
| Device Name: | UNDECIDED |
| Classification | System, Image Processing, Radiological |
| Applicant | SIEMENS MEDICAL SOLUTIONS USA, INC. 51 VALLEY STREAM PKWY. Malvern, PA 19355 -1406 |
| Contact | Richard K Andersen |
| Correspondent | Glenn Luchen UNDERWRITERS LABORATORIES, INC. 1285 WALT WHITMAN RD. Melville, NY 11747 |
| Product Code | LLZ |
| CFR Regulation Number | 892.2050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | Yes |
| Combination Product | No |
| Date Received | 2004-06-04 |
| Decision Date | 2004-07-30 |
| Summary: | summary |
Trademark Results [UNDECIDED]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
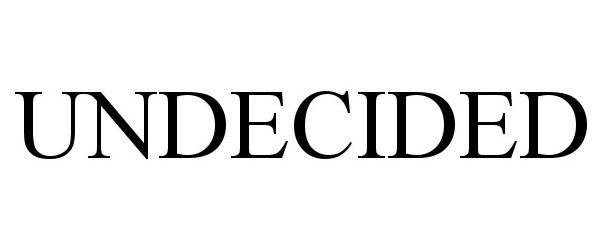 UNDECIDED 90648058 not registered Live/Pending |
Stephanie Gottlieb LLC 2021-04-15 |
 UNDECIDED 88856319 not registered Live/Pending |
Lee, Patrick 2020-04-01 |
 UNDECIDED 87488278 not registered Dead/Abandoned |
SIFUENETES, JEREMY 2017-06-14 |
 UNDECIDED 87079910 5140667 Live/Registered |
HENRY NICOLAS R. 2016-06-22 |
 UNDECIDED 86447842 5046154 Live/Registered |
Henry, Nicolas R 2014-11-07 |
 UNDECIDED 85587376 4918370 Live/Registered |
HENRY, NICOLAS R 2012-04-03 |
 UNDECIDED 79022788 3373192 Dead/Cancelled |
Antony Bruce Fenwick 2006-02-06 |
 UNDECIDED 79003845 3004592 Dead/Cancelled |
Antony Bruce Fenwick 2004-02-24 |
 UNDECIDED 76318490 not registered Dead/Abandoned |
DK Products, Inc. 2001-09-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.