GAMMAPLAN
System, Radiation Therapy, Radionuclide
ELEKTA INSTRUMENT AB
The following data is part of a premarket notification filed by Elekta Instrument Ab with the FDA for Gammaplan.
Pre-market Notification Details
| Device ID | K051022 |
| 510k Number | K051022 |
| Device Name: | GAMMAPLAN |
| Classification | System, Radiation Therapy, Radionuclide |
| Applicant | ELEKTA INSTRUMENT AB KUNGSTENSGATAN 18 PO BOX 7593 Stockholm, SE Se-103 93 |
| Contact | Peter Lowendahl |
| Correspondent | Peter Lowendahl ELEKTA INSTRUMENT AB KUNGSTENSGATAN 18 PO BOX 7593 Stockholm, SE Se-103 93 |
| Product Code | IWB |
| CFR Regulation Number | 892.5750 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2005-04-22 |
| Decision Date | 2005-06-01 |
| Summary: | summary |
Trademark Results [GAMMAPLAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
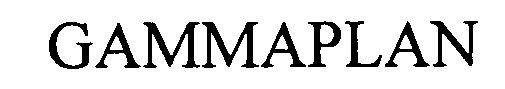 GAMMAPLAN 79013213 3136038 Dead/Cancelled |
Elekta AB (publ) 2005-02-02 |
 GAMMAPLAN 74624318 2078303 Live/Registered |
ELEKTA AB (PUBL) 1995-01-23 |
 GAMMAPLAN 74162714 not registered Dead/Abandoned |
ELEKTA INSTRUMENT S.A. 1991-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.