I-STOP
Mesh, Surgical, Synthetic, Urogynecologic, For Stress Urinary Incontinence, Retropubic Or Transobturator
CL MEDICAL
The following data is part of a premarket notification filed by Cl Medical with the FDA for I-stop.
Pre-market Notification Details
| Device ID | K051533 |
| 510k Number | K051533 |
| Device Name: | I-STOP |
| Classification | Mesh, Surgical, Synthetic, Urogynecologic, For Stress Urinary Incontinence, Retropubic Or Transobturator |
| Applicant | CL MEDICAL 28 AVENUE GENERAL DE GAULLE Sainte Foy Les Lyon, FR 69110 |
| Contact | Vincent Goria |
| Correspondent | Vincent Goria CL MEDICAL 28 AVENUE GENERAL DE GAULLE Sainte Foy Les Lyon, FR 69110 |
| Product Code | OTN |
| CFR Regulation Number | 878.3300 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2005-06-09 |
| Decision Date | 2005-08-11 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 03760151650338 | K051533 | 000 |
| 03760151650185 | K051533 | 000 |
| 03760151650093 | K051533 | 000 |
| 03760151650055 | K051533 | 000 |
| 03760151650048 | K051533 | 000 |
Trademark Results [I-STOP]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 I-STOP 85025600 not registered Dead/Abandoned |
MAZDA MOTOR CORPORATION 2010-04-28 |
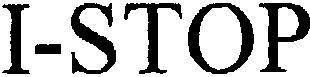 I-STOP 79013761 3196949 Live/Registered |
CL MEDICAL SARL 2005-03-24 |
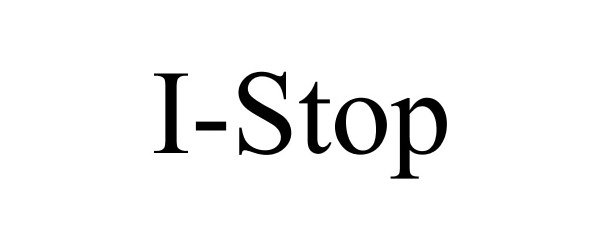 I-STOP 78766678 3235329 Dead/Cancelled |
Info Designs, Inc. 2005-12-05 |
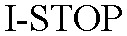 I-STOP 77027473 not registered Dead/Abandoned |
Jerry*Parker Inc. 2006-10-23 |
 I-STOP 76483274 3369785 Dead/Cancelled |
CARMANAH TECHNOLOGIES INC. 2003-01-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.