SIROLASER
Powered Laser Surgical Instrument
SIRONA DENTAL SYSTEMS GMBH
The following data is part of a premarket notification filed by Sirona Dental Systems Gmbh with the FDA for Sirolaser.
Pre-market Notification Details
| Device ID | K053161 |
| 510k Number | K053161 |
| Device Name: | SIROLASER |
| Classification | Powered Laser Surgical Instrument |
| Applicant | SIRONA DENTAL SYSTEMS GMBH FABRIKSTRASSE 31 Bensheim, DE D-64625 |
| Contact | Fritz Kolle |
| Correspondent | Stefan Preiss TUV AMERICA, INC. 1775 OLD HIGHWAY 8 New Brighton, MN 55112 -1891 |
| Product Code | GEX |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | Yes |
| Combination Product | No |
| Date Received | 2005-11-14 |
| Decision Date | 2006-01-18 |
| Summary: | summary |
Trademark Results [SIROLASER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
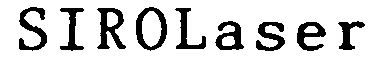 SIROLASER 79041416 3480883 Live/Registered |
Sirona Dental Systems GmbH 2007-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.