BF+ (PH)
Filler, Bone Void, Calcium Compound
LDR SPINE USA
The following data is part of a premarket notification filed by Ldr Spine Usa with the FDA for Bf+ (ph).
Pre-market Notification Details
| Device ID | K061462 |
| 510k Number | K061462 |
| Device Name: | BF+ (PH) |
| Classification | Filler, Bone Void, Calcium Compound |
| Applicant | LDR SPINE USA 4030 WEST BRAKER LANE SUITE 360 Austin, TX 78759 |
| Contact | Edward E Newton |
| Correspondent | Edward E Newton LDR SPINE USA 4030 WEST BRAKER LANE SUITE 360 Austin, TX 78759 |
| Product Code | MQV |
| CFR Regulation Number | 888.3045 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Special |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2006-05-26 |
| Decision Date | 2006-12-15 |
| Summary: | summary |
Trademark Results [BF+ (PH)]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
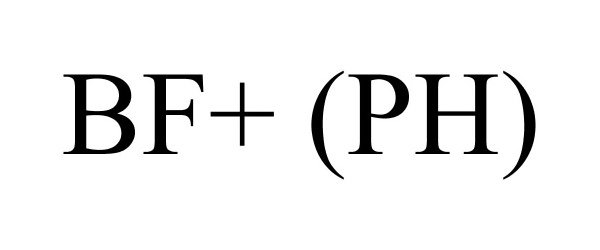 BF+ (PH) 78739474 3379420 Dead/Cancelled |
LDR MEDICAL, S.A.S. 2005-10-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.