ENDOGUIDE
Colonoscope And Accessories, Flexible/rigid
KMS MEDICAL LLC
The following data is part of a premarket notification filed by Kms Medical Llc with the FDA for Endoguide.
Pre-market Notification Details
| Device ID | K063654 |
| 510k Number | K063654 |
| Device Name: | ENDOGUIDE |
| Classification | Colonoscope And Accessories, Flexible/rigid |
| Applicant | KMS MEDICAL LLC 7290 SW 42ND STREET Miami, FL 33155 |
| Contact | Mario Arbesu |
| Correspondent | Mario Arbesu KMS MEDICAL LLC 7290 SW 42ND STREET Miami, FL 33155 |
| Product Code | FDF |
| CFR Regulation Number | 876.1500 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2006-12-08 |
| Decision Date | 2007-01-31 |
| Summary: | summary |
Trademark Results [ENDOGUIDE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
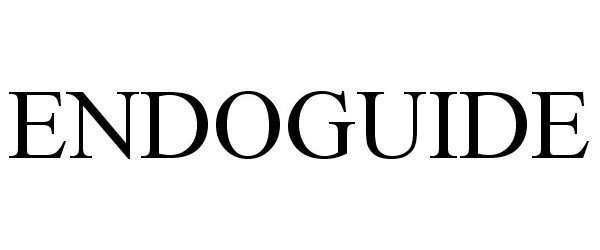 ENDOGUIDE 85106228 3976015 Live/Registered |
S.S. White Burs, Inc. 2010-08-12 |
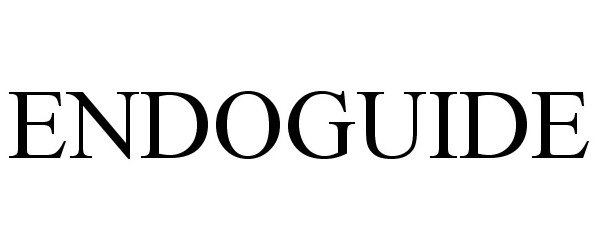 ENDOGUIDE 77029080 not registered Dead/Abandoned |
SYNTHEON, LLC 2006-10-25 |
 ENDOGUIDE 73797906 1577505 Dead/Cancelled |
LUXAR CORPORATION 1989-05-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.