PROGENIX
Bone Grafting Material, Human Source
MEDTRONIC SOFAMOR DANEK
The following data is part of a premarket notification filed by Medtronic Sofamor Danek with the FDA for Progenix.
Pre-market Notification Details
| Device ID | K082463 |
| 510k Number | K082463 |
| Device Name: | PROGENIX |
| Classification | Bone Grafting Material, Human Source |
| Applicant | MEDTRONIC SOFAMOR DANEK 1800 PYRAMID PLACE Memphis, TN 38132 |
| Contact | Ryan Massey |
| Correspondent | Ryan Massey MEDTRONIC SOFAMOR DANEK 1800 PYRAMID PLACE Memphis, TN 38132 |
| Product Code | NUN |
| CFR Regulation Number | 872.3930 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | Yes |
| Date Received | 2008-08-27 |
| Decision Date | 2008-11-10 |
| Summary: | summary |
Trademark Results [PROGENIX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
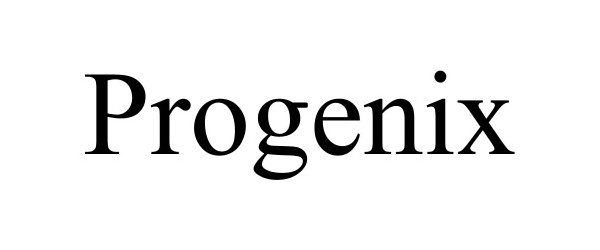 PROGENIX 98463197 not registered Live/Pending |
Hunt, Christian 2024-03-22 |
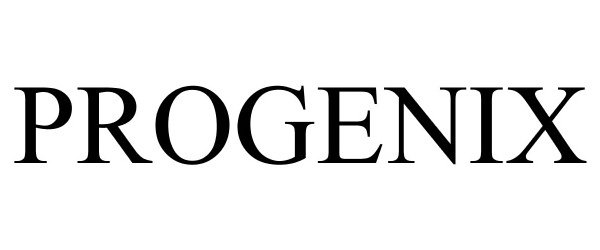 PROGENIX 87007945 not registered Dead/Abandoned |
Concept Laboratories, Inc. 2016-04-20 |
 PROGENIX 78199350 not registered Dead/Abandoned |
Medtronic, Inc. 2003-01-02 |
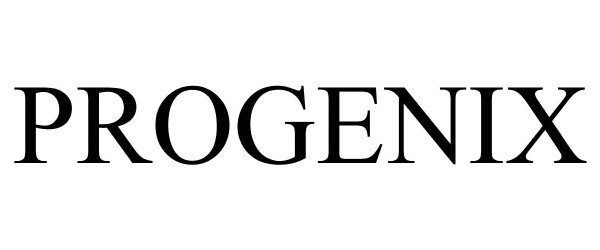 PROGENIX 77081784 3373649 Live/Registered |
Warsaw Orthopedic, Inc. 2007-01-12 |
 PROGENIX 75142407 not registered Dead/Abandoned |
OSIRIS THERAPEUTICS, INC. 1996-07-30 |
 PROGENIX 74583361 not registered Dead/Abandoned |
PROGENIX CORPORATION 1994-10-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.