C. DIFF QUIK CHEK COMPLETE
Reagents, Clostridium Difficile Toxin
TECHLAB, INC.
The following data is part of a premarket notification filed by Techlab, Inc. with the FDA for C. Diff Quik Chek Complete.
Pre-market Notification Details
| Device ID | K082499 |
| 510k Number | K082499 |
| Device Name: | C. DIFF QUIK CHEK COMPLETE |
| Classification | Reagents, Clostridium Difficile Toxin |
| Applicant | TECHLAB, INC. 2001 KRAFT DR. Blacksburg, VA 24060 -6358 |
| Contact | Charles Pennington |
| Correspondent | Charles Pennington TECHLAB, INC. 2001 KRAFT DR. Blacksburg, VA 24060 -6358 |
| Product Code | LLH |
| CFR Regulation Number | 866.2660 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2008-08-29 |
| Decision Date | 2009-03-26 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00857031002387 | K082499 | 000 |
| 00857031002370 | K082499 | 000 |
| 00857031002158 | K082499 | 000 |
| 00857031002059 | K082499 | 000 |
| 00857031002554 | K082499 | 000 |
Trademark Results [C. DIFF QUIK CHEK COMPLETE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
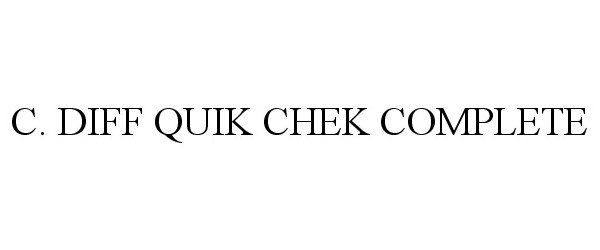 C. DIFF QUIK CHEK COMPLETE 77466957 3597654 Live/Registered |
TechLab, Inc. 2008-05-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.