ECOCEL
Bandage, Liquid
POLICHEM S.A.
The following data is part of a premarket notification filed by Polichem S.a. with the FDA for Ecocel.
Pre-market Notification Details
| Device ID | K083087 |
| 510k Number | K083087 |
| Device Name: | ECOCEL |
| Classification | Bandage, Liquid |
| Applicant | POLICHEM S.A. 7361 CALHOUN PLACE SUITE 500 Rockville, MD 20855 |
| Contact | Hari Nagaradona |
| Correspondent | Hari Nagaradona POLICHEM S.A. 7361 CALHOUN PLACE SUITE 500 Rockville, MD 20855 |
| Product Code | KMF |
| CFR Regulation Number | 880.5090 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2008-10-16 |
| Decision Date | 2009-08-07 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00343538510123 | K083087 | 000 |
Trademark Results [ECOCEL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
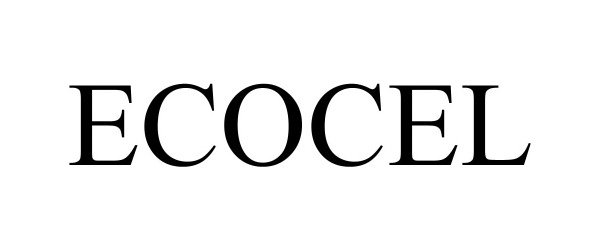 ECOCEL 98049114 not registered Live/Pending |
Willow Tex, LLC 2023-06-19 |
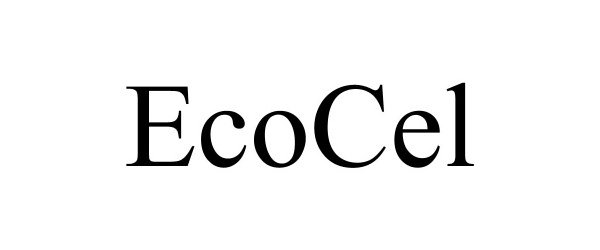 ECOCEL 88798698 not registered Live/Pending |
Earth Supplied Products 2020-02-14 |
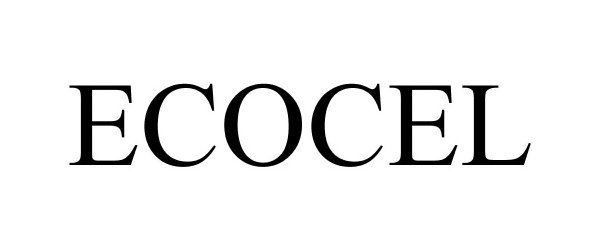 ECOCEL 87443753 not registered Dead/Abandoned |
Polichem S.A. 2017-05-10 |
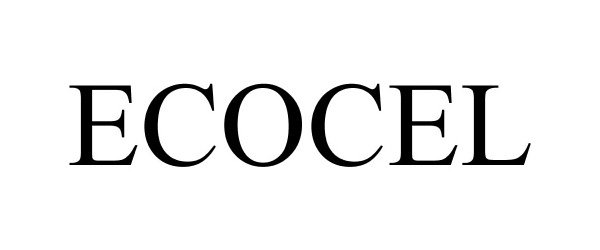 ECOCEL 86121232 not registered Dead/Abandoned |
Polichem S.A. 2013-11-18 |
 ECOCEL 85000421 not registered Dead/Abandoned |
Polichem S.A. 2010-03-29 |
 ECOCEL 77173184 3368544 Dead/Cancelled |
PRX Design Limited 2007-05-04 |
 ECOCEL 77173184 3368544 Dead/Cancelled |
Tohei Tsusho Company Limited 2007-05-04 |
 ECOCEL 75709720 2408038 Dead/Cancelled |
Preferred Power Technology, Inc. 1999-05-19 |
 ECOCEL 75325503 not registered Dead/Abandoned |
Preferred Power Technologies, Inc. 1997-07-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.