SIGNAL
Stethoscope, Electronic
ZARGIS MEDICAL CORP.
The following data is part of a premarket notification filed by Zargis Medical Corp. with the FDA for Signal.
Pre-market Notification Details
| Device ID | K090493 |
| 510k Number | K090493 |
| Device Name: | SIGNAL |
| Classification | Stethoscope, Electronic |
| Applicant | ZARGIS MEDICAL CORP. 2 RESEARCH WAY, 1ST FLOOR Princeton, NJ 08540 |
| Contact | John Kallassy |
| Correspondent | John Kallassy ZARGIS MEDICAL CORP. 2 RESEARCH WAY, 1ST FLOOR Princeton, NJ 08540 |
| Product Code | DQD |
| CFR Regulation Number | 870.1875 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2009-02-25 |
| Decision Date | 2009-08-07 |
| Summary: | summary |
Trademark Results [SIGNAL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SIGNAL 98875877 not registered Live/Pending |
Astec Industries, Inc. 2024-11-27 |
 SIGNAL 98875820 not registered Live/Pending |
Astec Industries, Inc. 2024-11-27 |
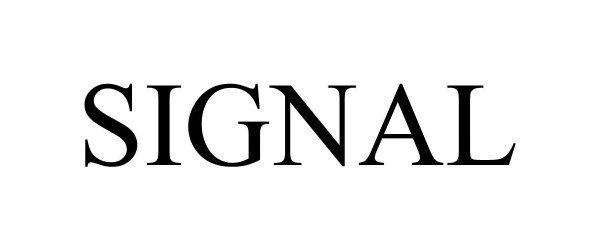 SIGNAL 98302951 not registered Live/Pending |
Signal Financial Federal Credit Union 2023-12-07 |
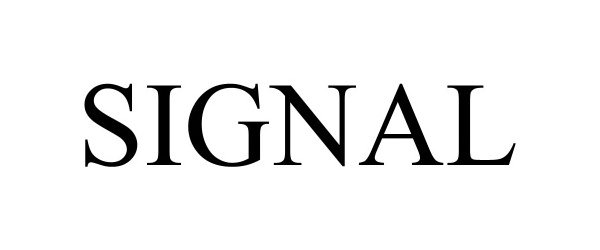 SIGNAL 98207398 not registered Live/Pending |
Little Giant Ladder Systems, LLC 2023-10-03 |
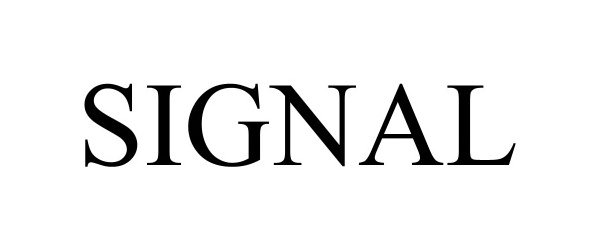 SIGNAL 97719671 not registered Live/Pending |
Ohserase Holdings, LLC 2022-12-15 |
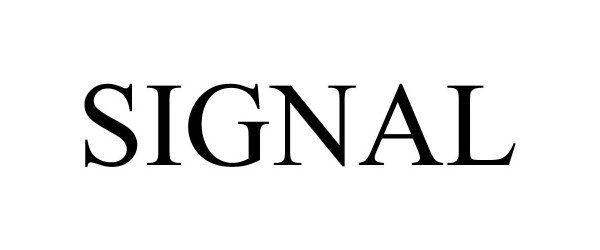 SIGNAL 97400593 not registered Live/Pending |
Vaughn, Jeffrey 2022-05-09 |
 SIGNAL 97225534 not registered Live/Pending |
Recognition Media, LLC 2022-01-18 |
 SIGNAL 97123205 not registered Live/Pending |
SignalPET, LLC 2021-11-12 |
 SIGNAL 97116588 not registered Live/Pending |
Plaid Inc. 2021-11-09 |
 SIGNAL 97099439 not registered Live/Pending |
FULL SPEED AUTOMATION Inc. 2021-10-29 |
 SIGNAL 97090822 not registered Live/Pending |
FUSE Oncology, Inc. 2021-10-25 |
 SIGNAL 90897577 not registered Live/Pending |
Signal 88, LLC 2021-08-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.