SEGMENT
System, Image Processing, Radiological
MEDVISO
The following data is part of a premarket notification filed by Medviso with the FDA for Segment.
Pre-market Notification Details
| Device ID | K090833 |
| 510k Number | K090833 |
| Device Name: | SEGMENT |
| Classification | System, Image Processing, Radiological |
| Applicant | MEDVISO THULEHEMSVAGEN 157 Lund, SE Se-22467 |
| Contact | Einar Heiberg |
| Correspondent | Einar Heiberg MEDVISO THULEHEMSVAGEN 157 Lund, SE Se-22467 |
| Product Code | LLZ |
| CFR Regulation Number | 892.2050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2009-03-26 |
| Decision Date | 2009-05-12 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 07350010100010 | K090833 | 000 |
Trademark Results [SEGMENT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SEGMENT 98364130 not registered Live/Pending |
Exemplis LLC 2024-01-18 |
 SEGMENT 90056325 not registered Live/Pending |
Exemplis LLC 2020-07-16 |
 SEGMENT 88653168 not registered Live/Pending |
Ste. Michelle Wine Estates Ltd. 2019-10-14 |
 SEGMENT 87879265 not registered Live/Pending |
Brandster GmbH 2018-04-16 |
 SEGMENT 87383821 not registered Live/Pending |
SEGMENT INTERNATIONAL LIMITED 2017-03-23 |
 SEGMENT 87377436 not registered Dead/Abandoned |
Apparatus LLC 2017-03-20 |
 SEGMENT 86568810 5209850 Live/Registered |
SEGMENT.IO, INC. 2015-03-18 |
 SEGMENT 77161814 3591952 Live/Registered |
BASF SE 2007-04-20 |
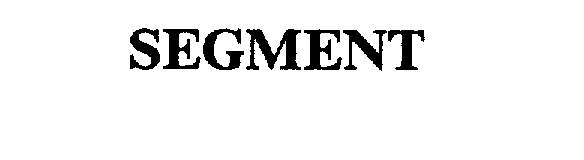 SEGMENT 75305182 2267620 Dead/Cancelled |
Steelcase Inc. 1997-06-09 |
 SEGMENT 75174762 2108883 Dead/Cancelled |
Segmed, Inc. 1996-09-12 |
 SEGMENT 74031983 1681390 Dead/Cancelled |
SEGMENT 1990-02-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.