TAKE 1
Material, Impression
KERR CORP.
The following data is part of a premarket notification filed by Kerr Corp. with the FDA for Take 1.
Pre-market Notification Details
| Device ID | K091613 |
| 510k Number | K091613 |
| Device Name: | TAKE 1 |
| Classification | Material, Impression |
| Applicant | KERR CORP. 1717 W. COLLINS AVENUE Orange, CA 92867 |
| Contact | Colleen Boswell |
| Correspondent | Colleen Boswell KERR CORP. 1717 W. COLLINS AVENUE Orange, CA 92867 |
| Product Code | ELW |
| CFR Regulation Number | 872.3660 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2009-06-03 |
| Decision Date | 2009-10-07 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 10841396118538 | K091613 | 000 |
| 10841396118248 | K091613 | 000 |
| 10841396118231 | K091613 | 000 |
| 10841396118224 | K091613 | 000 |
| 10841396118217 | K091613 | 000 |
| 10841396118200 | K091613 | 000 |
| 10841396118194 | K091613 | 000 |
| 10841396118187 | K091613 | 000 |
| 10841396118170 | K091613 | 000 |
| 10841396118163 | K091613 | 000 |
| 10841396118156 | K091613 | 000 |
| 10841396118149 | K091613 | 000 |
| 10841396118132 | K091613 | 000 |
| 10841396118125 | K091613 | 000 |
| 10841396118118 | K091613 | 000 |
| 10841396118101 | K091613 | 000 |
| 10841396118095 | K091613 | 000 |
| 10841396118255 | K091613 | 000 |
| 10841396118262 | K091613 | 000 |
| 10841396118279 | K091613 | 000 |
| 10841396118521 | K091613 | 000 |
| 10841396118514 | K091613 | 000 |
| 10841396118507 | K091613 | 000 |
| 10841396118491 | K091613 | 000 |
| 10841396118484 | K091613 | 000 |
| 10841396118392 | K091613 | 000 |
| 10841396118385 | K091613 | 000 |
| 10841396118378 | K091613 | 000 |
| 10841396118361 | K091613 | 000 |
| 10841396118354 | K091613 | 000 |
| 10841396118347 | K091613 | 000 |
| 10841396118323 | K091613 | 000 |
| 10841396118316 | K091613 | 000 |
| 10841396118309 | K091613 | 000 |
| 10841396118293 | K091613 | 000 |
| 10841396118286 | K091613 | 000 |
| 00841396118401 | K091613 | 000 |
Trademark Results [TAKE 1]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
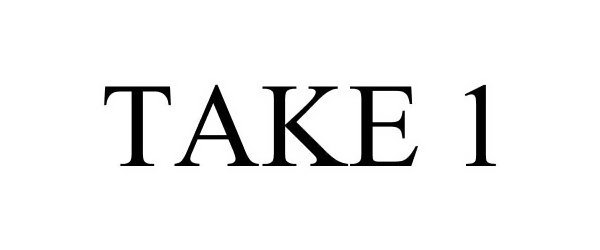 TAKE 1 85107222 4068240 Live/Registered |
Elite Brands Inc. 2010-08-13 |
 TAKE 1 78883883 not registered Dead/Abandoned |
Take 1 Technologies, LLC 2006-05-15 |
 TAKE 1 75525778 2352682 Dead/Cancelled |
Kerr Corporation 1998-07-27 |
 TAKE 1 75165667 2203488 Dead/Cancelled |
AUDIO PRODUCTS INTERNATIONAL CORP. 1996-09-12 |
 TAKE 1 73772328 1651357 Dead/Cancelled |
TONG YANG POLYESTER CO., LTD. 1988-12-30 |
 TAKE 1 73311986 1209819 Dead/Cancelled |
Take 1 Sportswear, Inc. 1981-05-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.