E-PULSE
Stimulator, Electro-acupuncture
MEDEVICE CORPORATION
The following data is part of a premarket notification filed by Medevice Corporation with the FDA for E-pulse.
Pre-market Notification Details
| Device ID | K091875 |
| 510k Number | K091875 |
| Device Name: | E-PULSE |
| Classification | Stimulator, Electro-acupuncture |
| Applicant | MEDEVICE CORPORATION 11 NE 15TH AVE Pompano Beach, FL 33060 |
| Contact | Tzvi Milshtein |
| Correspondent | Tzvi Milshtein MEDEVICE CORPORATION 11 NE 15TH AVE Pompano Beach, FL 33060 |
| Product Code | BWK |
| CFR Regulation Number | 510(k) Premarket Notification [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2009-06-23 |
| Decision Date | 2009-12-07 |
| Summary: | summary |
Trademark Results [E-PULSE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
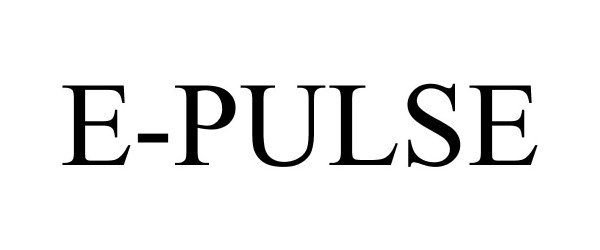 E-PULSE 87882854 5909159 Live/Registered |
Actuant Corporation 2018-04-18 |
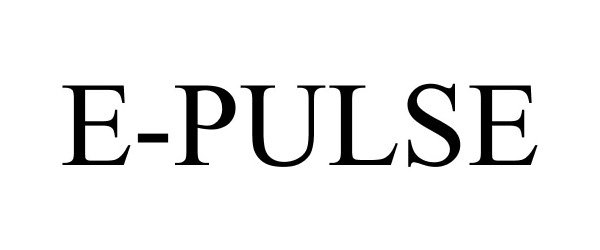 E-PULSE 86487698 4847808 Live/Registered |
Enovative Technologies, LLC 2014-12-22 |
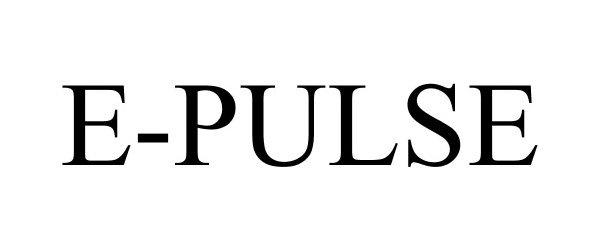 E-PULSE 85464525 not registered Dead/Abandoned |
Glow Industries, Inc. 2011-11-04 |
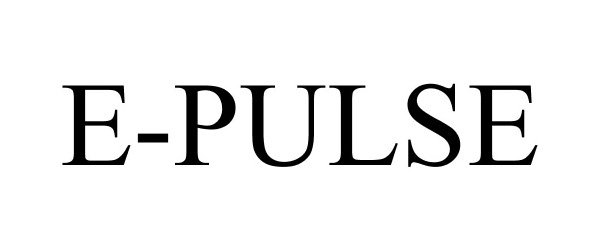 E-PULSE 85089563 3925655 Live/Registered |
JOHNSON & JOHNSON 2010-07-21 |
 E-PULSE 75721822 not registered Dead/Abandoned |
SUPPLY CHAIN SOLUTIONS, INC. 1999-06-03 |
 E-PULSE 75657620 2428850 Dead/Cancelled |
Vie-Core Technologies, Inc. 1999-03-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.