DYSIS
Colposcope (and Colpomicroscope)
FORTH PHOTONICS HELLAS SA
The following data is part of a premarket notification filed by Forth Photonics Hellas Sa with the FDA for Dysis.
Pre-market Notification Details
| Device ID | K092433 |
| 510k Number | K092433 |
| Device Name: | DYSIS |
| Classification | Colposcope (and Colpomicroscope) |
| Applicant | FORTH PHOTONICS HELLAS SA 690 CANTON ST, SUITE 302 Westwood, MA 02090 |
| Contact | Juan Carlos Serna |
| Correspondent | Juan Carlos Serna FORTH PHOTONICS HELLAS SA 690 CANTON ST, SUITE 302 Westwood, MA 02090 |
| Product Code | HEX |
| CFR Regulation Number | 884.1630 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2009-08-07 |
| Decision Date | 2011-03-10 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 15060477110185 | K092433 | 000 |
| 15060477110062 | K092433 | 000 |
| 05060477110027 | K092433 | 000 |
| 15060477110017 | K092433 | 000 |
| 15060477110000 | K092433 | 000 |
| 05060477110263 | K092433 | 000 |
Trademark Results [DYSIS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DYSIS 97089827 not registered Live/Pending |
NIDA IÃ VE DIS TICARET ANONIM SIRKETI 2021-10-24 |
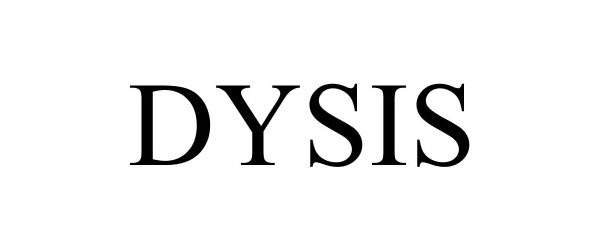 DYSIS 86946080 5612301 Live/Registered |
Dysis Medical Limited 2016-03-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.