EMBRACE
System, X-ray, Mammographic
IZI MEDICAL PRODUCTS INC.
The following data is part of a premarket notification filed by Izi Medical Products Inc. with the FDA for Embrace.
Pre-market Notification Details
| Device ID | K101373 |
| 510k Number | K101373 |
| Device Name: | EMBRACE |
| Classification | System, X-ray, Mammographic |
| Applicant | IZI MEDICAL PRODUCTS INC. 7020 TUDSBURY RD. Baltimore, MD 21244 |
| Contact | Helen Shafer |
| Correspondent | Helen Shafer IZI MEDICAL PRODUCTS INC. 7020 TUDSBURY RD. Baltimore, MD 21244 |
| Product Code | IZH |
| CFR Regulation Number | 892.1710 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2010-05-17 |
| Decision Date | 2010-09-15 |
| Summary: | summary |
Trademark Results [EMBRACE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EMBRACE 98764157 not registered Live/Pending |
ASTI Manufacturing Corp, Inc. 2024-09-23 |
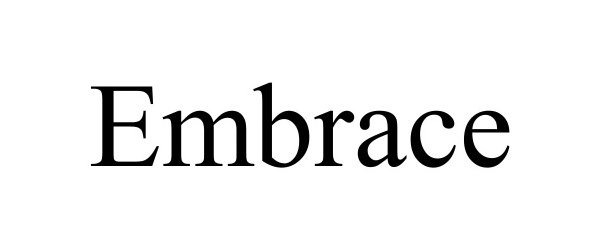 EMBRACE 98715527 not registered Live/Pending |
Embrace Global 2024-08-24 |
 EMBRACE 98597399 not registered Live/Pending |
DMF LLC 2024-06-12 |
 EMBRACE 98257711 not registered Live/Pending |
Jiangsu Embrace Science&Technology Development Co., Ltd. 2023-11-06 |
 EMBRACE 98012543 not registered Live/Pending |
Sales & Product Solutions, Inc. 2023-05-24 |
 EMBRACE 97642792 not registered Live/Pending |
New Pride Corp 2022-10-21 |
 EMBRACE 97621309 not registered Live/Pending |
C2C Performance Lifestyle, LLC 2022-10-06 |
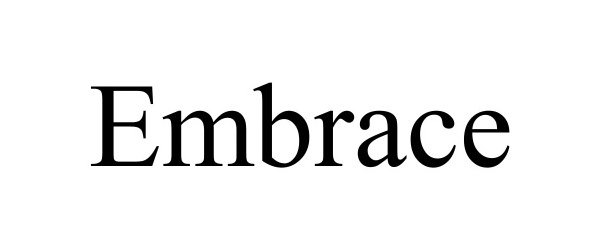 EMBRACE 97519178 not registered Live/Pending |
SilverGull Games LLC 2022-07-25 |
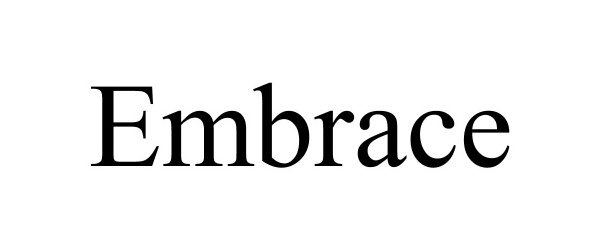 EMBRACE 97407254 not registered Live/Pending |
AgXplore International LLC 2022-05-12 |
 EMBRACE 97282877 not registered Live/Pending |
Heartland Recreational Vehicles, LLC 2022-02-24 |
 EMBRACE 97169569 not registered Live/Pending |
Carefree Pharmacy, Inc. 2021-12-13 |
 EMBRACE 97023935 not registered Live/Pending |
Twin Rivers Paper Company LLC 2021-09-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.