IBAG
Collector, Urine, (and Accessories) For Indwelling Catheter
FUTURE PATH MEDICAL, LLC
The following data is part of a premarket notification filed by Future Path Medical, Llc with the FDA for Ibag.
Pre-market Notification Details
| Device ID | K110518 |
| 510k Number | K110518 |
| Device Name: | IBAG |
| Classification | Collector, Urine, (and Accessories) For Indwelling Catheter |
| Applicant | FUTURE PATH MEDICAL, LLC 8870 RAVELLO CT Naples, FL 34114 |
| Contact | Daniel Kamm |
| Correspondent | Daniel Kamm FUTURE PATH MEDICAL, LLC 8870 RAVELLO CT Naples, FL 34114 |
| Product Code | KNX |
| CFR Regulation Number | 876.5250 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2011-02-23 |
| Decision Date | 2011-06-24 |
| Summary: | summary |
Trademark Results [IBAG]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
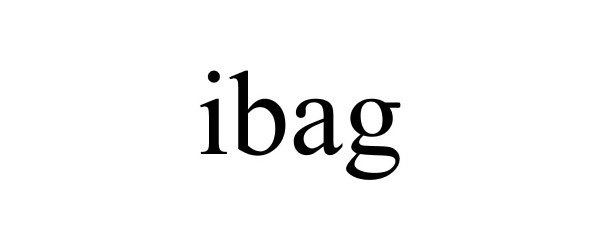 IBAG 85601454 not registered Dead/Abandoned |
H2 Galaxy Corp. 2012-04-18 |
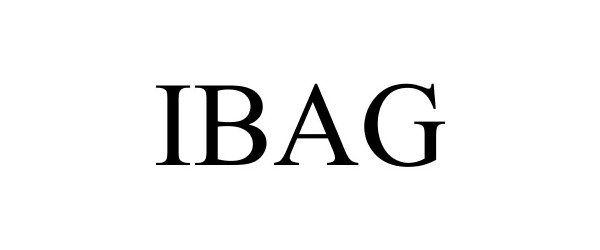 IBAG 85410338 not registered Dead/Abandoned |
Future Path Medical Holding Company, LLC 2011-08-30 |
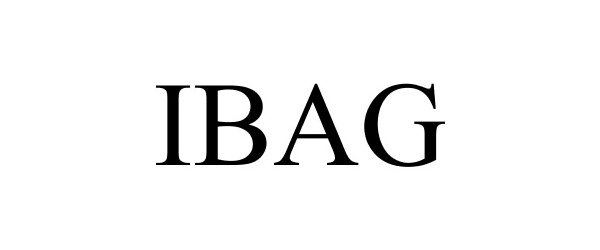 IBAG 85372469 4333508 Live/Registered |
Spiritual & Personal Growth Trust 2011-07-15 |
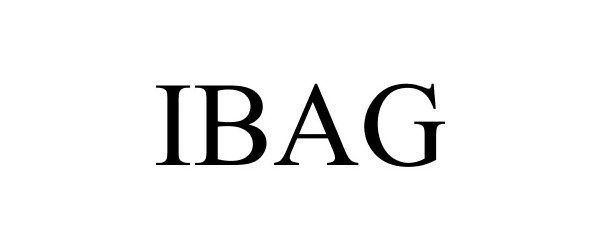 IBAG 85231173 not registered Dead/Abandoned |
Cryovac, Inc. 2011-02-01 |
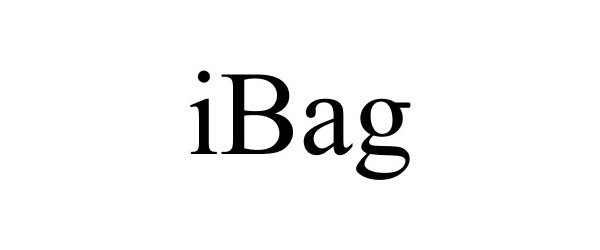 IBAG 85112026 4011332 Dead/Cancelled |
CARTER, JACQUELINE 2010-08-20 |
 IBAG 79189175 5352505 Live/Registered |
ifoodbag AB 2016-04-20 |
 IBAG 79104714 4181435 Live/Registered |
IBAG Switzerland AG 2011-09-22 |
 IBAG 78099271 2921412 Dead/Cancelled |
Cryovac, Inc. 2001-12-19 |
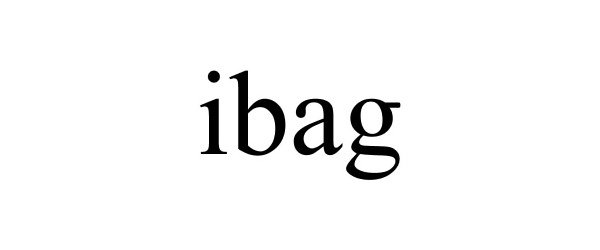 IBAG 77086416 not registered Dead/Abandoned |
DonJuan Gross 2007-01-19 |
 IBAG 75811994 2436581 Dead/Cancelled |
IBAG Switzerland AG 1999-09-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.