PRISMAFLEX
Dialyzer, High Permeability With Or Without Sealed Dialysate System
GAMBRO RENAL PRODUCTS, INC.
The following data is part of a premarket notification filed by Gambro Renal Products, Inc. with the FDA for Prismaflex.
Pre-market Notification Details
| Device ID | K110823 |
| 510k Number | K110823 |
| Device Name: | PRISMAFLEX |
| Classification | Dialyzer, High Permeability With Or Without Sealed Dialysate System |
| Applicant | GAMBRO RENAL PRODUCTS, INC. 14143 DENVER WEST PARKWAY SUITE 400 Lakewood, CO 80401 |
| Contact | Kae Miller |
| Correspondent | Kae Miller GAMBRO RENAL PRODUCTS, INC. 14143 DENVER WEST PARKWAY SUITE 400 Lakewood, CO 80401 |
| Product Code | KDI |
| CFR Regulation Number | 876.5860 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2011-03-24 |
| Decision Date | 2011-06-17 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 07332414105266 | K110823 | 000 |
Trademark Results [PRISMAFLEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRISMAFLEX 78861703 not registered Dead/Abandoned |
Gambro Lundia AB 2006-04-14 |
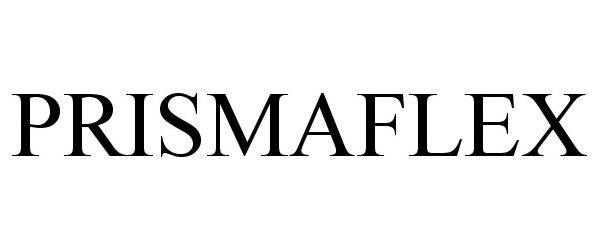 PRISMAFLEX 78859340 not registered Dead/Abandoned |
Gambro Lundia AB 2006-04-11 |
 PRISMAFLEX 78513902 3102098 Live/Registered |
Gambro Lundia AB 2004-11-09 |
 PRISMAFLEX 78272350 2979008 Live/Registered |
Gambro Lundia AB 2003-07-09 |
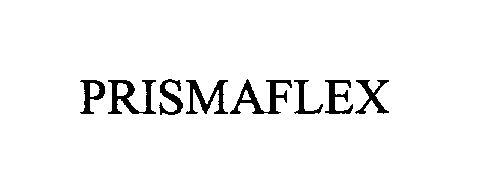 PRISMAFLEX 76290339 not registered Dead/Abandoned |
RIGIFLEX INTERNATIONAL 2001-07-26 |
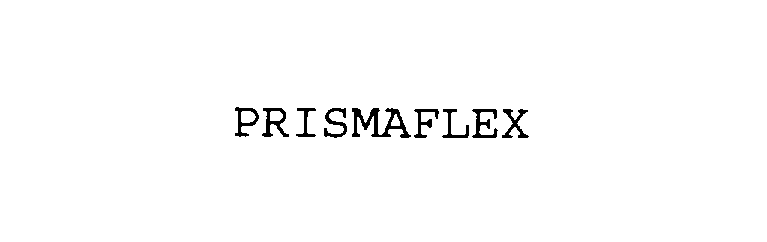 PRISMAFLEX 76115953 2698334 Live/Registered |
Prismaflex USA, Inc 2000-08-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.