E-PASS
Adaptor, Stopcock, Manifold, Fitting, Cardiopulmonary Bypass
SYNEXMED(SHENZHEN)COMPANY LIMITED
The following data is part of a premarket notification filed by Synexmed(shenzhen)company Limited with the FDA for E-pass.
Pre-market Notification Details
| Device ID | K113148 |
| 510k Number | K113148 |
| Device Name: | E-PASS |
| Classification | Adaptor, Stopcock, Manifold, Fitting, Cardiopulmonary Bypass |
| Applicant | SYNEXMED(SHENZHEN)COMPANY LIMITED B-11/F,Z Z Y BUILD.,TAOHUA RD. FUTIAN FREE TRADE ZONE Shenzhen, CN 518038 |
| Contact | Tony Zhang |
| Correspondent | Tony Zhang SYNEXMED(SHENZHEN)COMPANY LIMITED B-11/F,Z Z Y BUILD.,TAOHUA RD. FUTIAN FREE TRADE ZONE Shenzhen, CN 518038 |
| Product Code | DTL |
| CFR Regulation Number | 870.4290 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2011-10-24 |
| Decision Date | 2012-02-28 |
| Summary: | summary |
Trademark Results [E-PASS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
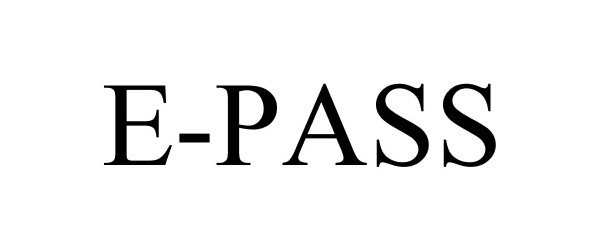 E-PASS 88499937 not registered Live/Pending |
Central Florida Expressway Authority 2019-07-03 |
 E-PASS 76037557 not registered Dead/Abandoned |
Revelation. L.L.C 2000-05-01 |
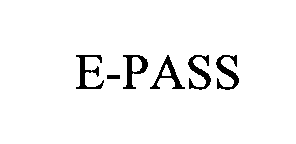 E-PASS 75709584 2495394 Live/Registered |
Dallas Area Rapid Transit 1999-05-19 |
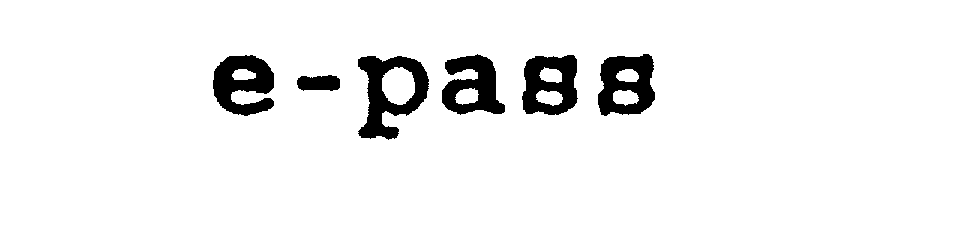 E-PASS 75139825 2304460 Dead/Cancelled |
Hennige, Hartmut 1996-07-25 |
 E-PASS 74448680 not registered Dead/Abandoned |
ORLANDO/ORANGE COUNTY EXPRESSWAY AUTHORITY 1993-10-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.