Z-LOOK3 EVO SLM
Implant, Endosseous, Root-form
Z-Systems AG
The following data is part of a premarket notification filed by Z-systems Ag with the FDA for Z-look3 Evo Slm.
Pre-market Notification Details
| Device ID | K120793 |
| 510k Number | K120793 |
| Device Name: | Z-LOOK3 EVO SLM |
| Classification | Implant, Endosseous, Root-form |
| Applicant | Z-Systems AG 11234 EL CAMINO REAL STE: 200 San Diego, CA 92024 |
| Contact | Linda Schulz |
| Correspondent | Linda Schulz Z-Systems AG 11234 EL CAMINO REAL STE: 200 San Diego, CA 92024 |
| Product Code | DZE |
| CFR Regulation Number | 872.3640 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2012-03-15 |
| Decision Date | 2012-12-21 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 07640166760153 | K120793 | 000 |
| 07640166760009 | K120793 | 000 |
| 07640166760016 | K120793 | 000 |
| 07640166760023 | K120793 | 000 |
| 07640166760030 | K120793 | 000 |
| 07640166760047 | K120793 | 000 |
| 07640166760054 | K120793 | 000 |
| 07640166760061 | K120793 | 000 |
| 07640166760078 | K120793 | 000 |
| 07640166760085 | K120793 | 000 |
| 07640166760092 | K120793 | 000 |
| 07640166760108 | K120793 | 000 |
| 07640166760115 | K120793 | 000 |
| 07640166760122 | K120793 | 000 |
| 07640166760139 | K120793 | 000 |
| 07640166760146 | K120793 | 000 |
| 07640166769491 | K120793 | 000 |
Trademark Results [Z-LOOK3 EVO SLM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
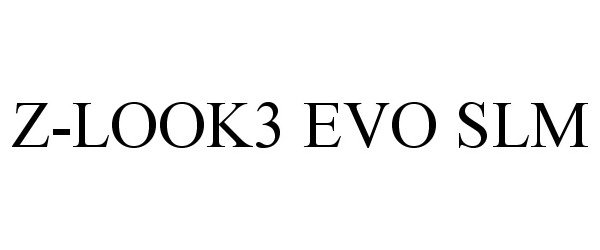 Z-LOOK3 EVO SLM 85641721 not registered Dead/Abandoned |
Z-Systems Schweiz AG 2012-06-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.