STR GRAFT
Mesh, Surgical, Absorbable, Orthopaedics, Reinforcement Of Tendon
SOFT TISSUE REGENERATION, INC.
The following data is part of a premarket notification filed by Soft Tissue Regeneration, Inc. with the FDA for Str Graft.
Pre-market Notification Details
| Device ID | K121216 |
| 510k Number | K121216 |
| Device Name: | STR GRAFT |
| Classification | Mesh, Surgical, Absorbable, Orthopaedics, Reinforcement Of Tendon |
| Applicant | SOFT TISSUE REGENERATION, INC. 815 IRIS LANE Vero Beach, FL 32963 |
| Contact | Robert A Poggie |
| Correspondent | Robert A Poggie SOFT TISSUE REGENERATION, INC. 815 IRIS LANE Vero Beach, FL 32963 |
| Product Code | OWW |
| CFR Regulation Number | 878.3300 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2012-04-23 |
| Decision Date | 2012-11-21 |
| Summary: | summary |
Trademark Results [STR GRAFT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
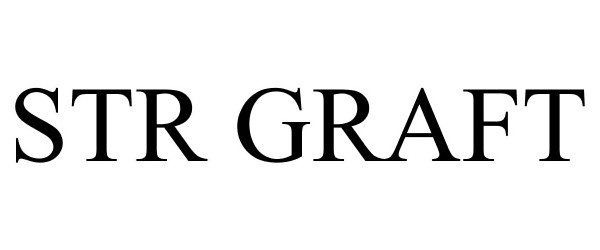 STR GRAFT 86531780 not registered Dead/Abandoned |
Soft Tissue Regeneration, Inc. 2015-02-11 |
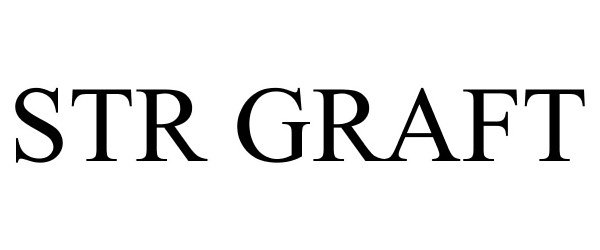 STR GRAFT 85336709 not registered Dead/Abandoned |
SOFT TISSUE REGNERATION, INC. 2011-06-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.