VPULSE
Sleeve, Limb, Compressible
COTHERA, LLC
The following data is part of a premarket notification filed by Cothera, Llc with the FDA for Vpulse.
Pre-market Notification Details
| Device ID | K122640 |
| 510k Number | K122640 |
| Device Name: | VPULSE |
| Classification | Sleeve, Limb, Compressible |
| Applicant | COTHERA, LLC 1331 H STREET NW 12TH FLOOR Washington, DC 20005 |
| Contact | Justin Eggleton |
| Correspondent | Justin Eggleton COTHERA, LLC 1331 H STREET NW 12TH FLOOR Washington, DC 20005 |
| Product Code | JOW |
| CFR Regulation Number | 870.5800 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2012-08-29 |
| Decision Date | 2013-02-22 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00672736065303 | K122640 | 000 |
| 00672736064825 | K122640 | 000 |
| 00672736064948 | K122640 | 000 |
| 00672736064955 | K122640 | 000 |
| 00672736064962 | K122640 | 000 |
| 00672736064979 | K122640 | 000 |
| 00672736064986 | K122640 | 000 |
| 00672736065051 | K122640 | 000 |
| 00672736065068 | K122640 | 000 |
| 00672736065099 | K122640 | 000 |
| 00672736065112 | K122640 | 000 |
| 00672736065150 | K122640 | 000 |
| 00672736065167 | K122640 | 000 |
| 00672736064818 | K122640 | 000 |
Trademark Results [VPULSE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
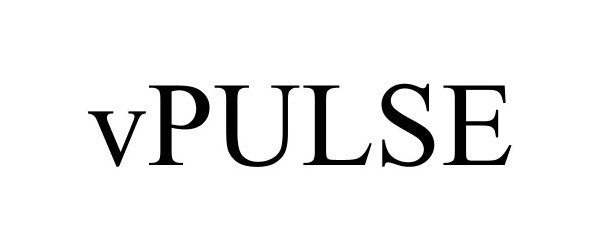 VPULSE 98520934 not registered Live/Pending |
vcfo Holdings, Inc. 2024-04-26 |
 VPULSE 85756048 4632905 Live/Registered |
BREG, INC. 2012-10-17 |
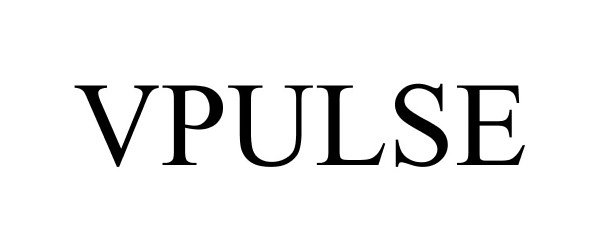 VPULSE 85622201 4369804 Live/Registered |
BREG, INC. 2012-05-10 |
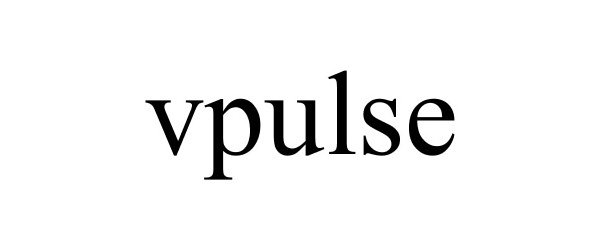 VPULSE 85520373 4198247 Dead/Cancelled |
VELODYNE ACOUSTICS, LLC 2012-01-19 |
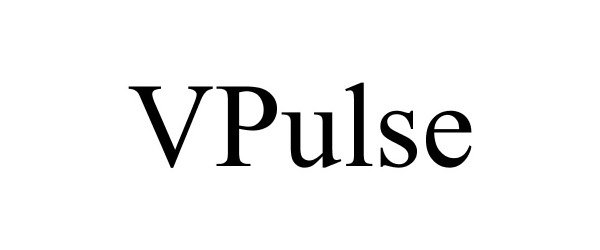 VPULSE 85026590 not registered Dead/Abandoned |
Ventus Networks 2010-04-29 |
 VPULSE 79140846 4762311 Live/Registered |
Vollmer Werke Maschinenfabrik; GmbH 2013-10-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.