XTOOL
Arthroscope
OUROBOROS MEDICAL
The following data is part of a premarket notification filed by Ouroboros Medical with the FDA for Xtool.
Pre-market Notification Details
| Device ID | K122861 |
| 510k Number | K122861 |
| Device Name: | XTOOL |
| Classification | Arthroscope |
| Applicant | OUROBOROS MEDICAL 47757 FREMONT BLVD Fremont, CA 94538 |
| Contact | Shelley Trimm |
| Correspondent | Shelley Trimm OUROBOROS MEDICAL 47757 FREMONT BLVD Fremont, CA 94538 |
| Product Code | HRX |
| CFR Regulation Number | 888.1100 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2012-09-18 |
| Decision Date | 2012-12-07 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 10705034507439 | K122861 | 000 |
| 10705034507422 | K122861 | 000 |
| 10705034507415 | K122861 | 000 |
| 10705034507408 | K122861 | 000 |
| 10705034507392 | K122861 | 000 |
| 10705034507385 | K122861 | 000 |
| 10705034507378 | K122861 | 000 |
| 10705034507361 | K122861 | 000 |
Trademark Results [XTOOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XTOOL 98488086 not registered Live/Pending |
Makeblock Co., Ltd. 2024-04-08 |
 XTOOL 98488049 not registered Live/Pending |
Makeblock Co., Ltd. 2024-04-08 |
 XTOOL 98467477 not registered Live/Pending |
SHENZHEN XTOOLTECH INTELLIGENT CO., LTD. 2024-03-25 |
 XTOOL 98342134 not registered Live/Pending |
Makeblock Co., Ltd. 2024-01-04 |
 XTOOL 90223683 not registered Live/Pending |
MAKEBLOCK CO., LTD. 2020-09-30 |
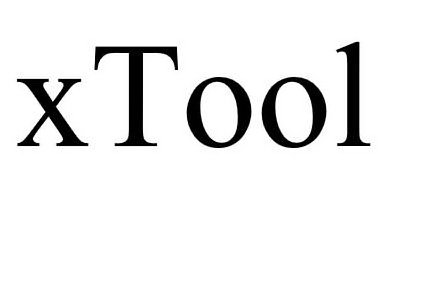 XTOOL 90223646 not registered Live/Pending |
MAKEBLOCK CO., LTD. 2020-09-29 |
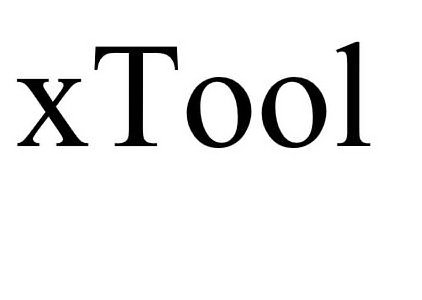 XTOOL 90223607 not registered Live/Pending |
MAKEBLOCK CO., LTD. 2020-09-29 |
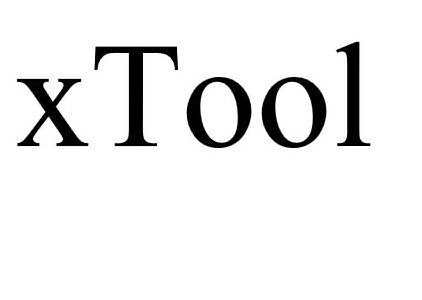 XTOOL 90223586 not registered Live/Pending |
MAKEBLOCK CO., LTD. 2020-09-29 |
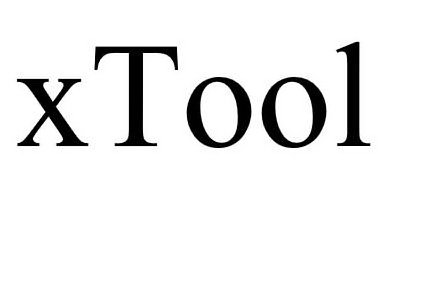 XTOOL 90223570 not registered Live/Pending |
MAKEBLOCK CO., LTD. 2020-09-29 |
 XTOOL 85806025 4479907 Live/Registered |
XTOOLTECH LLC 2012-12-18 |
 XTOOL 85722545 4548366 Live/Registered |
DEPUY SYNTHES, INC. 2012-09-06 |
 XTOOL 79247167 not registered Dead/Abandoned |
Shenzhen Xtooltech Co., Ltd. 2018-09-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.